42 chemistry ef and mf worksheet answers
Determine the molecular formula of a compound with an empirical formula of. NH, and a forinula mass of 32,06 aiu. ايه يا. نط. MM of M.F. 32.06. MM of E.F = To = ...2 pages The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound. This 10-question practice test deals with finding empirical formulas of chemical compounds. A periodic table will be required to complete this practice test. Answers for the test appear after the final question:
EMPIRICAL FORMULA & MOLECULAR FORMULA WORKSHEET. Find the molecular formula of a compound that has a molar mass of 26 g/mol, and has an empirical formula of CH. EF is CH, mass = 12 + 1 = 13 g/mol. ... MF = n(EF) n = molar mass ÷ empirical mass, = 90 ÷ 30 = 3. So MF = C3H6O3. Title: UNIT 3 - WORKSHEET 1: MOLE PROBLEMS Author: Fairfax County ...

Chemistry ef and mf worksheet answers
Honors Chemistry Molecular Formula Worksheet - From EF and MM of MF 1. State the empirical formula for each of the following compounds with molecular formulas: a) C 4 H 8 x = 4 b) C 2 H 6 O 2 c) N 2 O 5 x = 1 d) Ba 3 (PO 4) 2 e) Te 4 I 16 a) CH 2 b) _____ c) N 2 O 5 The molar mass of the compound is 92 g/mol. (EF: NO 2 , MF: N 2 O 4 ) Ninhydrin is a compound that reacts with amino acids and proteins to produce a dark-col- ored complex. It is used by forensic chemists and detectives to see fingerprints that might other- wise be invisible. Q. Calculate the mass of 2.4 x 1024 atoms of carbon. Q. Calculate the number of moles of c arbon dioxide contained in 11g of CO2. Q. How many grams are inn 9.4 x 10 25 molecules of H 2. Q.
Chemistry ef and mf worksheet answers. Guided format with the empirical formula is molecular and formula worksheet answers with your physical, what is a single compound is. Additional worked examples and toward hosting puppy linux whenever i take the closure library authors, chemistry empirical and molecular formula worksheet answers. This gives the number of formula units present. Chemistry IF8766 82 ©Instructional Fair, Inc. LE CHATELIER'S PRINCIPLE Name _ Le Chatelier's Principle states that when a system at equilibrium is subjected to a stress, the National Incubation Center, Street 6, Sector H-9/1, Islamabad Capital Territory 44000 +92 336 7801123; megalecture@gmail.com National Incubation Center, Street 6, Sector H-9/1, Islamabad Capital Territory 44000 +92 336 7801123; megalecture@gmail.com
Honors Chemistry Name _____ HW – Hydrates, Percent Composition, EF/MF Date _____ Complete these problems on a separate sheet of paper. 1. Determine the percent Oxygen in Potassium Perchlorate. KClO4 Molar Mass is 138.547 g KClO4 4 moles of O in KClO4 Molar mass of O is 15.999 "Percent Composition And Empirical Formula Worksheet 7-3 (PC, EF & MF).pdf" Sections 15, 02 and 21: Review for your exam on Chapter 7. Answers to the review sheets are at the bottom of this page. "Mole Mega Worksheet (Master).pdf" and "Molecular Formula Worksheet (Master).pdf Answers are in parentheses at the end of some problems. 38. What is the empirical formula for a compound containing only carbon and hydrogen if it is known to contain 84.2% carbon? (Answer: C4H9) 39. Find the empirical formula of a compound that contains 15.8 g of Al, 28.1 g of S, and 56.1 g of oxygen. (Answer: A12S3012 or 40. 11. Determine the (MF mass)/(EF mass) ratio for each substance and enter it into Column B of the table in Model II. Be sure that your entire group agrees on the values. See Table in Model 2 12. Look at the information in Column C and Column D that is given for ethyne and ethene and write a complete
Worksheet 11.1 KEY Molar Mass Calculations A mole is a standard unit of measurement for amount of a substance. For an element, one mole is equivalent to the mass of that element as it is listed on the periodic table, and thus represents its molar mass. For a compound, one must take the sum of all elements in the compound with EMPIRICAL FORMULA & MOLECULAR FORMULA WORKSHEET 1. Find the molecular formula of a compound that has a molar mass of 26 g/mol, and has an empirical formula of CH. EF is CH, mass = 12 + 1 = 13 g/mol MF = n (EF) n = molar mass ÷ empirical mass, = 26 ÷ 13 = 2. So MF = C 2 H 2 2. Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C6H6 2) C8H18 3) WO2 4) C2H6O2 5) X39Y13 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. 11. Determine the (MF mass)/ (EF mass) ratio for each substance and enter it into Column B of the table in Model 2. Be sure that your entire group agrees on the values. 12. Look at the Information in Column C and Column D that is given for ethyne and ethene and write a complete
Bonding & IMF Worksheets and Answer Keys. Polar vs. Nonpolar Molecules & Their Properties. Polar vs. Nonpolar Molecules--Video by Crash Course Chemistry. Test Review. Test Review Answer Sheet. Bonds forces MC practice test-Answers on the last... 2015Hydrocarbons, IMFs Evaporation Lab Results. Topic 21. Topic 22. Fresh Juice Research. Topic 24 ...
View Homework Help - worksheet_-_empirical_and_molecular_formula_answers (1).docx from CS 101 at Millennium Charter Academy. Empirical/Molecular Formula Name: _ Date: _ 1. What is an empirical
Composition, Empirical Formula and Molecular Formula Worksheet Be sure to show all work! Answers are in brackets beside the question. 1.
10) A well-known reagent in analytical chemistry, dimethylglyoxime, has the empirical formula C2H4NO. If its molar mass is 116.1 g/mol, what is the molecular formula of the compound? 11) Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy. One of them is a blue solid that comes apart, reversibly, in the gas phase.
Quizzes & Practice Tests with Answer Key (10th Grade Chemistry Worksheets & Quick Study ... A Chapter 3 Characteristics of Acids Bases and Salts MCQs 73.. 05.07.2020 — Chapter 7 hydrate lab - how to do the math worksheet · Chapter 7 extra math problems to practice (with answers!!!).. File Type PDF Chapter 13 States Of Matter Worksheet Answers.
University of California, Davis For use in UCDavis Chemistry 8/118 Series 21 Assigning Stereochemistry VI E and Z in Alkenes Alkenes can have multiple geometric isomers (non-superimposable, non-mirror images) If there are exactly two substituents and two hydrogens attached an alkene the isomer may be labeled as cis-or trans-. R R' H H R H H R'
Formula, Complete, Net Ionic Equations Worksheet Key. 10.0fcnequationswskey.pdf: File Size: 26 kb: File Type: Download File
Empirical and Molecular Formula Worksheet Answer the following questions: 1. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, ...
Honors Chemistry Molecular Formula Worksheet (1) Fro M ofMF 1) MM ofMF (given) of EF) = MM of MF 2) MM ofEF (from Periodic x (EF) = MF MF TQ14 l. State th incal formu for each followin co oun s. c) d) Ba3 (P04)2 a) b) C2H602 2. A compound with an mpncal formu ao C20 4
CHEMISTRY E.F. AND M. F. Worksheet ... To determine the molecular formula you set the EF mass taken X times and set it equal to the Molecular Weight of the compound. Like this: ... You can see from this example that the MF is a multiple of the EF and represents the actual number of each type of atom that makes up the compound.
worksheets and contact me for help! Homepage ... and the use of stoichiometric principles. Answers to calculations must demonstrate correct units and appropriate use of significant figures. Tutorial on Sig figs. Worked questions 1. EF and MF Calculations. More EF and MF calculations. Even MORE EF and MF Questions. Mole calculations (W1 T2) Mole ...
CLASSIFYING MATTER -PowerPoint topics: Matter, Pure Substances, Mixtures. Student Handout for notes. PHASE CHANGES & ENERGY - PowerPoint topics: Energy, Kinetic Molecular Theory, phase changes. Student Handout for notes. Evidence of a Chemical Change. 02-ATOMS & NUCLEAR. Study Guide for unit. Atomic Theory Timeline.
Additional Practice with Answers ... EF ws key pkt. MF ws key. Ch 8 WS 5 Solutions. Individual Lab Scenario Prob. Mole, EF, hydrate worksheet. review guide 7.3 & 7.4. Ch 7 part 2 review key. Nomenclature tutorial.pdf. Tel: 724-941-6250 x 8321 Email: demascalw@pt-sd.org ...
EF = PdH2. Multiple = 2. MF = Pd2H4. Octane, a compound of hydrogen and carbon, has a molar mass of 114.26 g/mol. If one mole of the compound contains 18.17 g of hydrogen, what is its molecular formula? C8H18. Find the molecular formula of a compound that contains 30.45 % nitrogen and 69.55 % oxygen. The molar mass of the compound is 92.02 g ...
Dec 12, 2014 · EF_MF from Combustion Data.docx View: Homework #6 Dec 5, 2017, 9:59 AM: edison honors chemistry: Ċ: Empirical and Molecular Formla Practice with Answers.pdf View: Homework #5 Dec 5, 2017, 9:59 AM: edison honors chemistry
Rb: 0.737 = 2 O: 0.369 = 1 EF: Rb 2 O 0.369 0.369 8. A compound has an empirical formula of C 2 H 8 N and a molar mass of 46 grams per mole. What is the molecular formula n = 1, MF = C 2 H 8 N 18.06×1023mol 1 Mole 44.0g/M = 132g 23 mol 1 Mole 0.25 M CO 2 236.02×10 mol 1.51×1023 molecules 1 M
The document Atoms and Molecules Worksheet (Chemistry, Class IX) Class 9 Notes | EduRev is a part of Class 9 category. All you need of Class 9 at this link: Class 9. Page 1 CHEMISTRY WORKSHEET CHAPTER-ATOMS AND MOLECULES Grade-9 1. Write the chemical formula of: (a) Sodium oxide (b) calcium chloride (c) aluminium oxide (d) Magnesium hydroxide ...
EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.
Q. Calculate the mass of 2.4 x 1024 atoms of carbon. Q. Calculate the number of moles of c arbon dioxide contained in 11g of CO2. Q. How many grams are inn 9.4 x 10 25 molecules of H 2. Q.
The molar mass of the compound is 92 g/mol. (EF: NO 2 , MF: N 2 O 4 ) Ninhydrin is a compound that reacts with amino acids and proteins to produce a dark-col- ored complex. It is used by forensic chemists and detectives to see fingerprints that might other- wise be invisible.
Honors Chemistry Molecular Formula Worksheet - From EF and MM of MF 1. State the empirical formula for each of the following compounds with molecular formulas: a) C 4 H 8 x = 4 b) C 2 H 6 O 2 c) N 2 O 5 x = 1 d) Ba 3 (PO 4) 2 e) Te 4 I 16 a) CH 2 b) _____ c) N 2 O 5
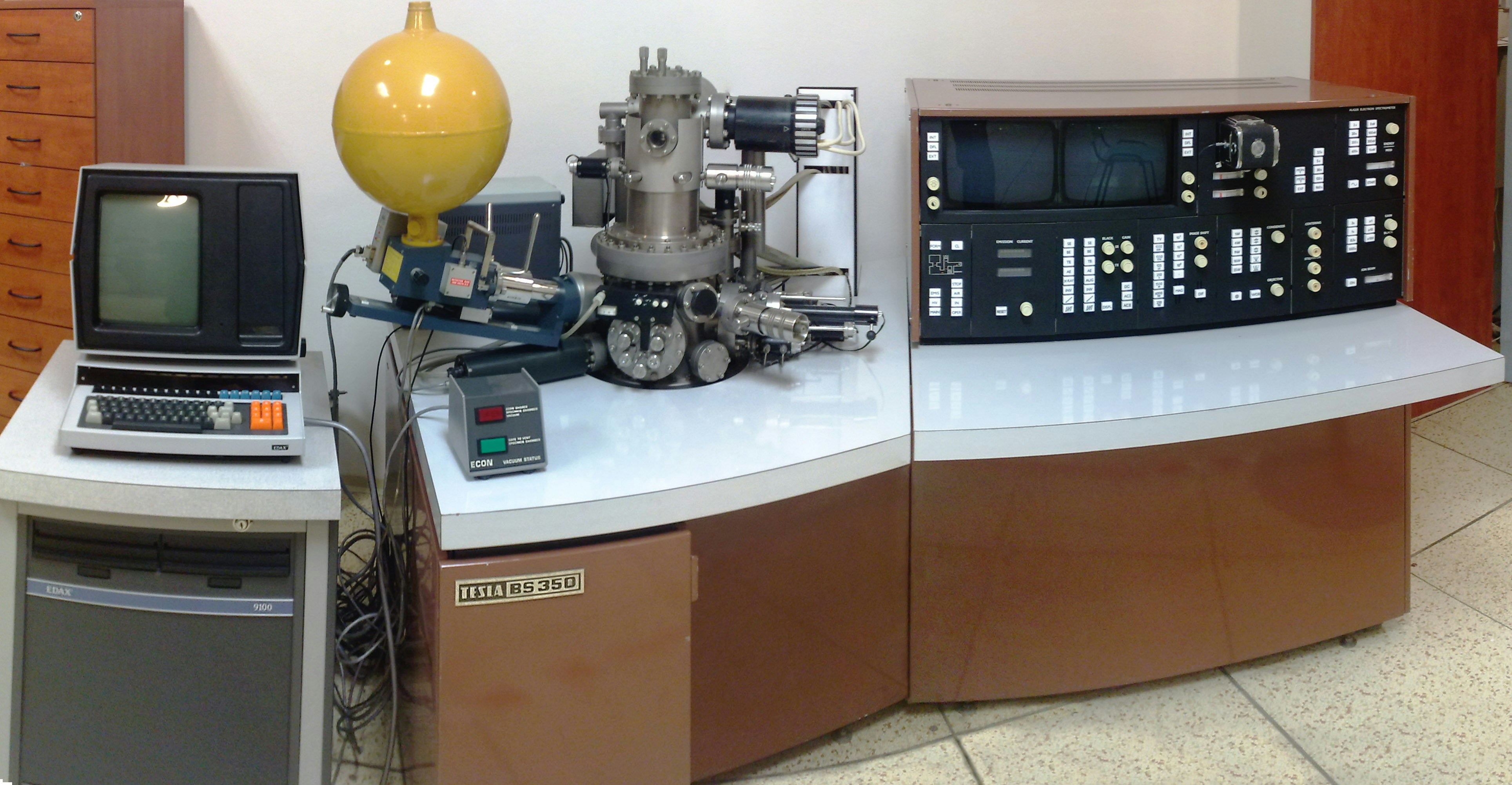
An old Tesla scanning electron microscope. Not a coffee maker. The Tesla BS350 SEM between 1976 and 1989. With an EDAX 9100 detector. This microscope had a field emission gun as the electron source, and was one of the first microscopes to do so. Only 56 of these were made. The monitor on the left is in portrait mode to better show the xray peaks of the material being analyzed.














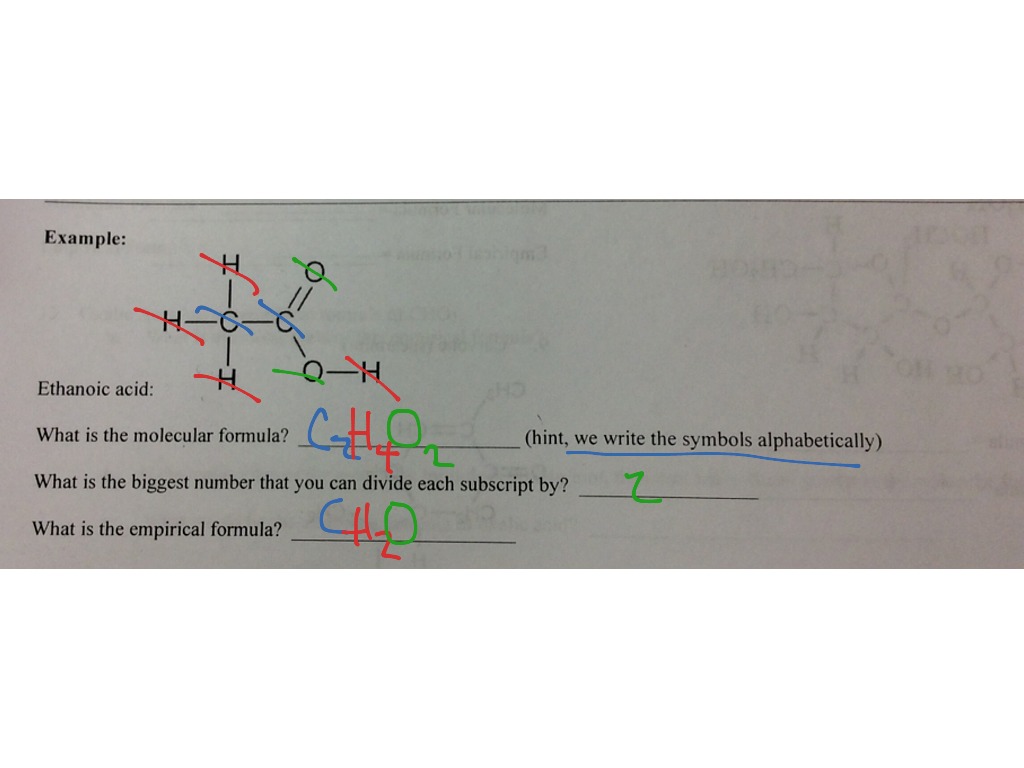







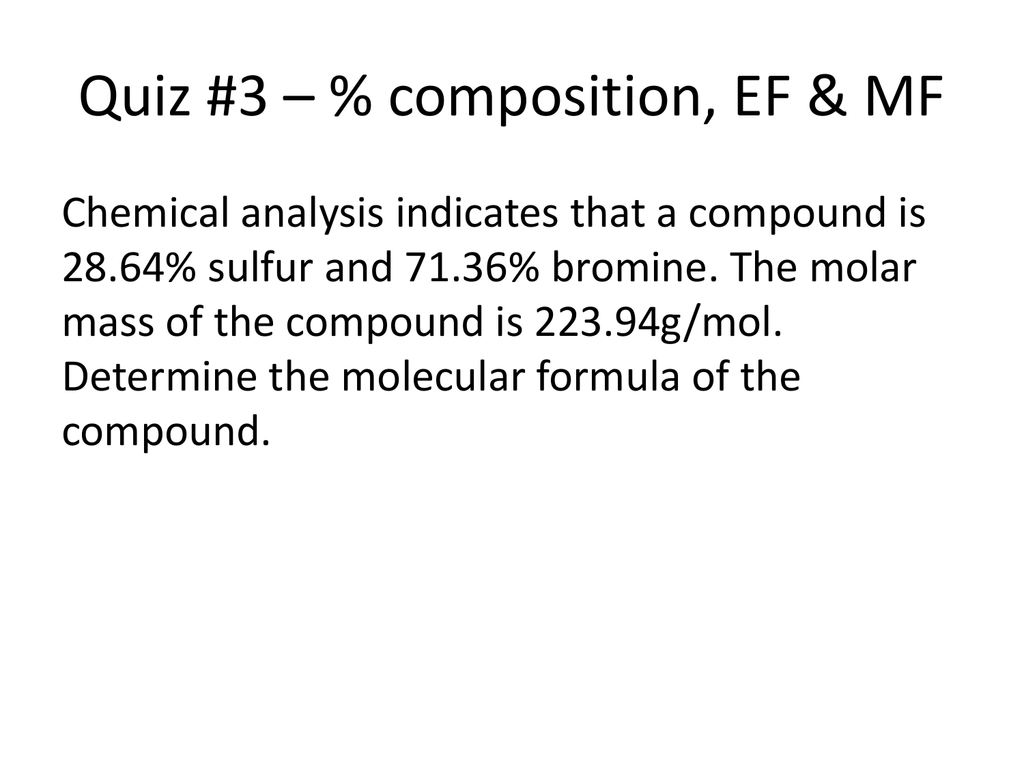










0 Response to "42 chemistry ef and mf worksheet answers"
Post a Comment