40 isotopes and average atomic mass worksheet
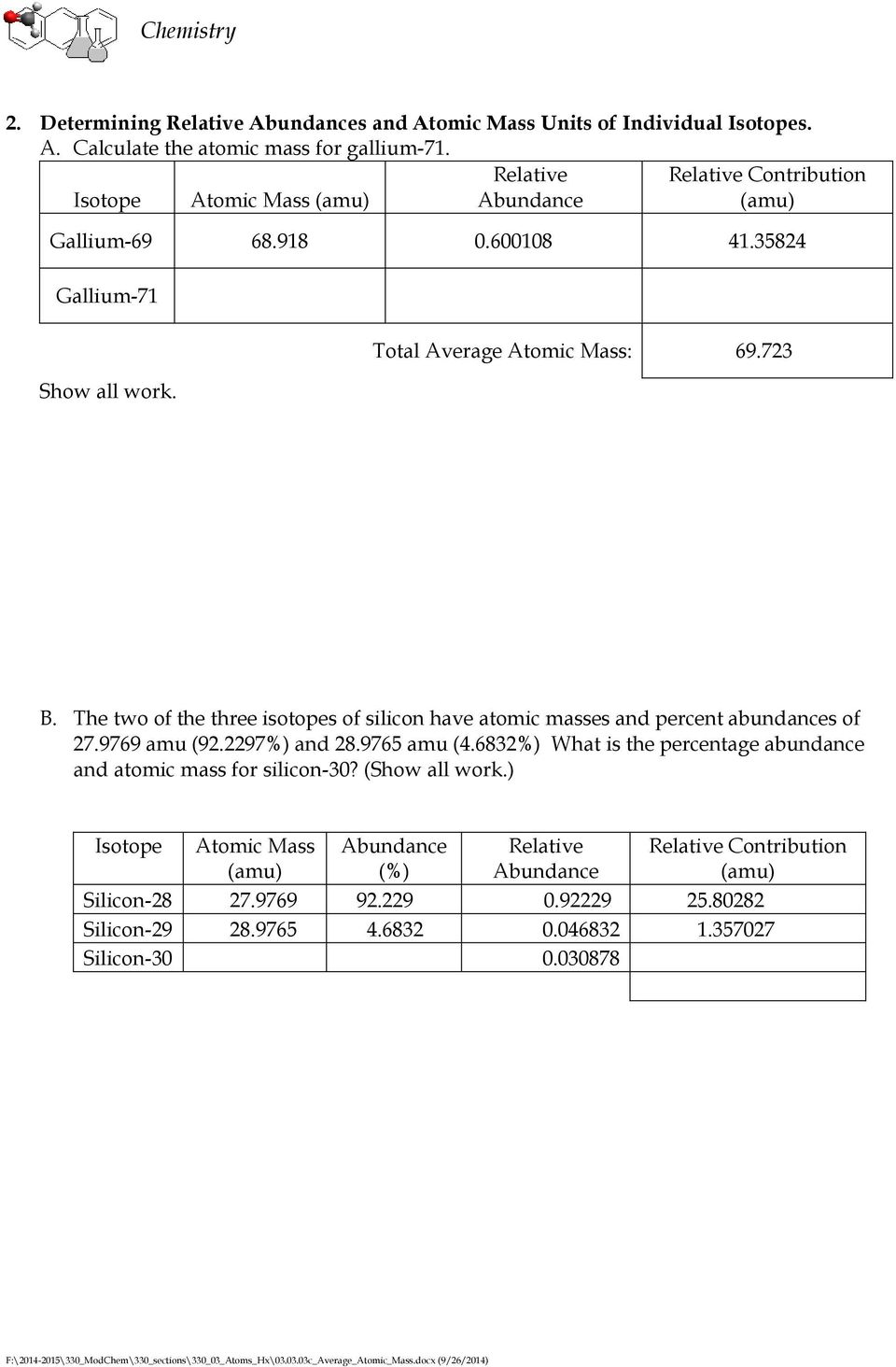
PDF Isotopes and Average Atomic Mass - FISD The periodic table reports the average atomic mass, which is a weighted average of all isotopes of b oron. 8. A certain element has two isotopes. One isotope, which has a percent abundance of 72.15% has a mass of 84.9118 amu. The other isotope has a mass of 86.9092 amu. a. Calculate the average atomic mass of this element to three decimal places PDF Isotope Worksheet Answer Key - ISD 622 1. Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55) 55.85 12 14 (03115) GoaYlG) 13,3 + (H 5) + ( , 3 a) 2. How many neutrons does Zn-66 have? mass 3. Give the hyphen notation for this atom, p=76, e= 76, n= 116 11, is 11 on Is (93, This is 4.
study.com › skill › learnHow to Calculate Average Atomic Mass - Study.com Atomic mass: The atomic mass is the mass of an atom, and is obtained by finding the average mass of the isotopes of a given element. Isotopes: Isotopes are the atoms with the same number of ...
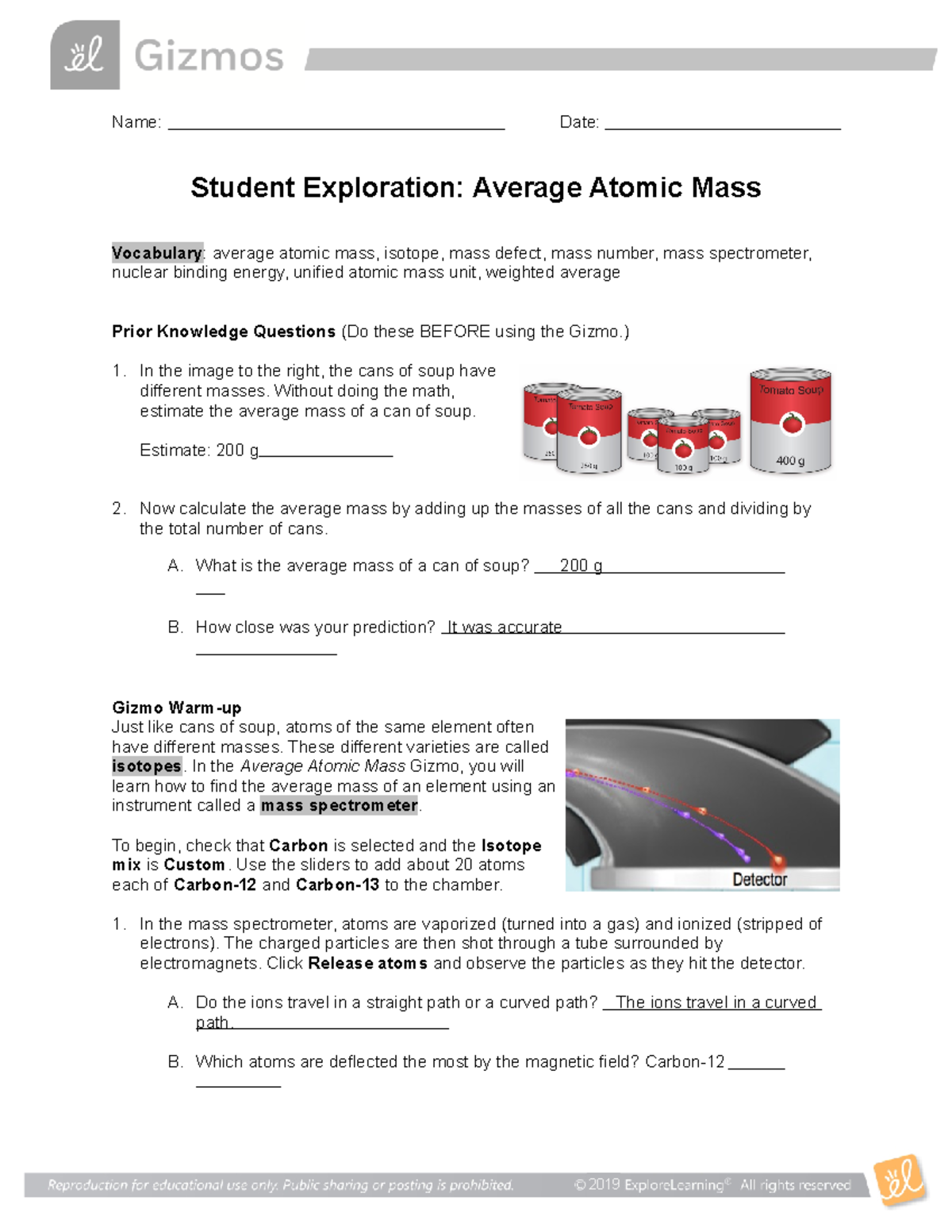
Isotopes and average atomic mass worksheet
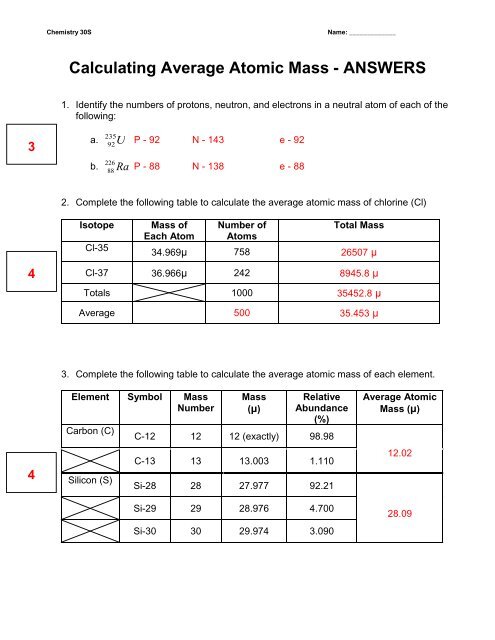
PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. Isotopes And Average Atomic Mass Chemistry Worksheet ... Isotopes And Average Atomic Mass Chemistry Worksheet - Worksheets are an inexpensive, high-impact source. In several circumstances, these have actually been revealed to be effective in quickening student discovering. It is among the most extensively used training help worldwide today, as well as for good factor. courses.lumenlearning.com › cheminter › chapterCalculating Atomic Mass | Chemistry for Non-Majors Sample Problem: Calculating Atomic Mass . Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine. Step 1: List the known and unknown quantities and plan the problem. Known . chlorine-35: atomic mass = 34.969 amu and % abundance = 75.77%
Isotopes and average atomic mass worksheet. Quiz & Worksheet - Isotopes and Average Atomic Mass ... Isotopes and average atomic mass, as concepts, allow for the specific discussion of elements and their atoms, and this quiz/worksheet combo will help you test your understanding of these concepts. phet.colorado.edu › sims › htmlIsotopes and Atomic Mass - PhET Interactive Simulations Isotopes and Atomic Mass - PhET Interactive Simulations › relative-atomic-massIsotopes & Relative Atomic Mass (solutions, examples, videos) Atomic Mass: Introduction. What is atomic mass? It is a weighed average of the different isotopes of an element. It is sometimes referred to as atomic weight, relative atomic mass, or average atomic mass. We look at how to calculate and determine the weighed average of elements using atomic mass units. Show Video Lesson PDF Isotopes and average atomic mass practice worksheet answers Worksheet Answer Key. 16 Best Images Of Atomic Structure Worksheet Answer Chart … , Atomic Structure Element Symbol Atomic Mass (Common Number Number Isotope) Hydrogen H 1 1 Helium He 2 4 Lithium Li Nitrogen N Oxygen O Silicon Si Krypton Kr Lead Pb Uranium U Plutonium Pu Isotopes Element 3 7 8 14 36 82 92 94 7 14 16 28 84 207 238 242 Number Of Particles In The Atom. 11 Best
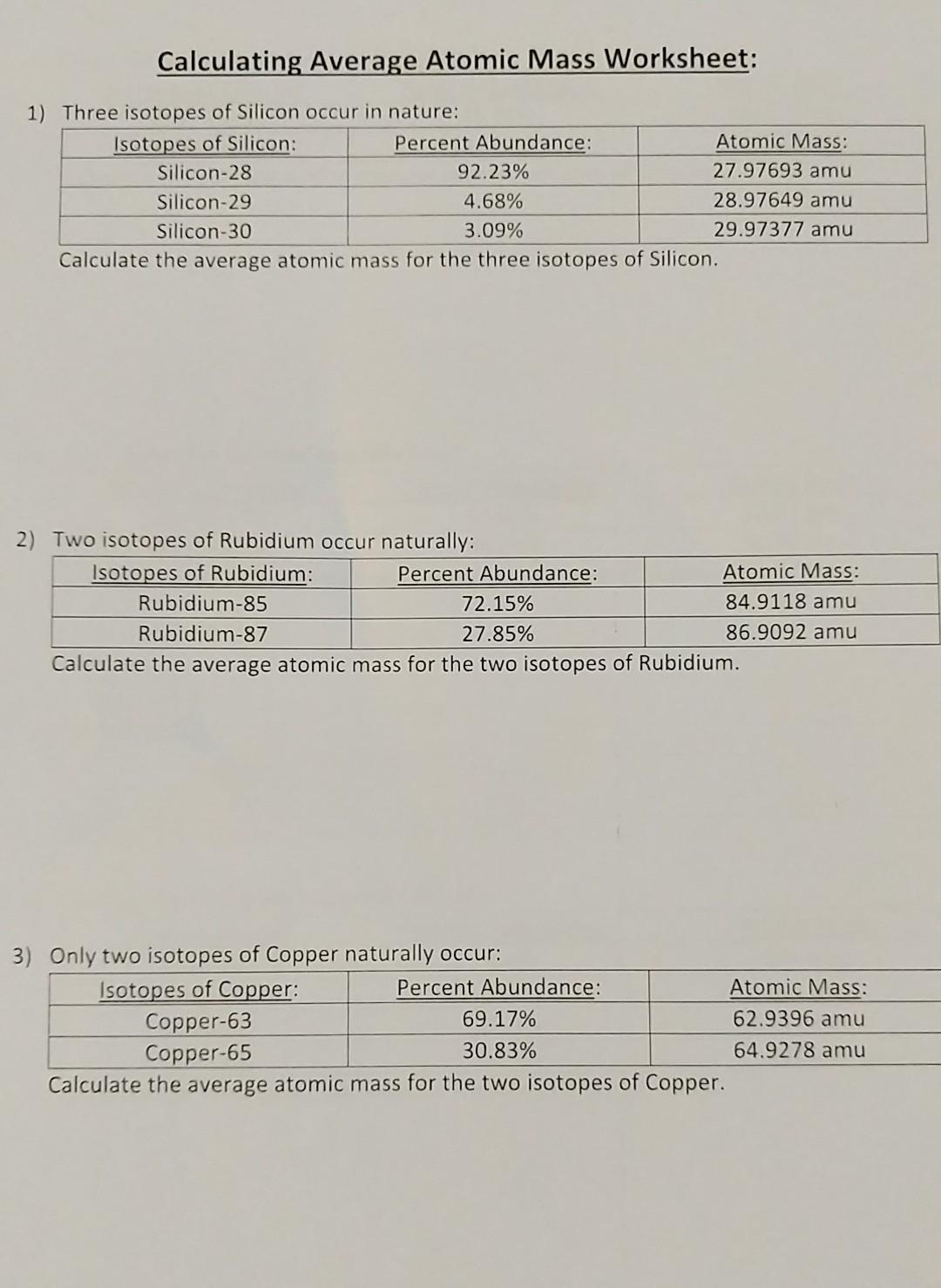
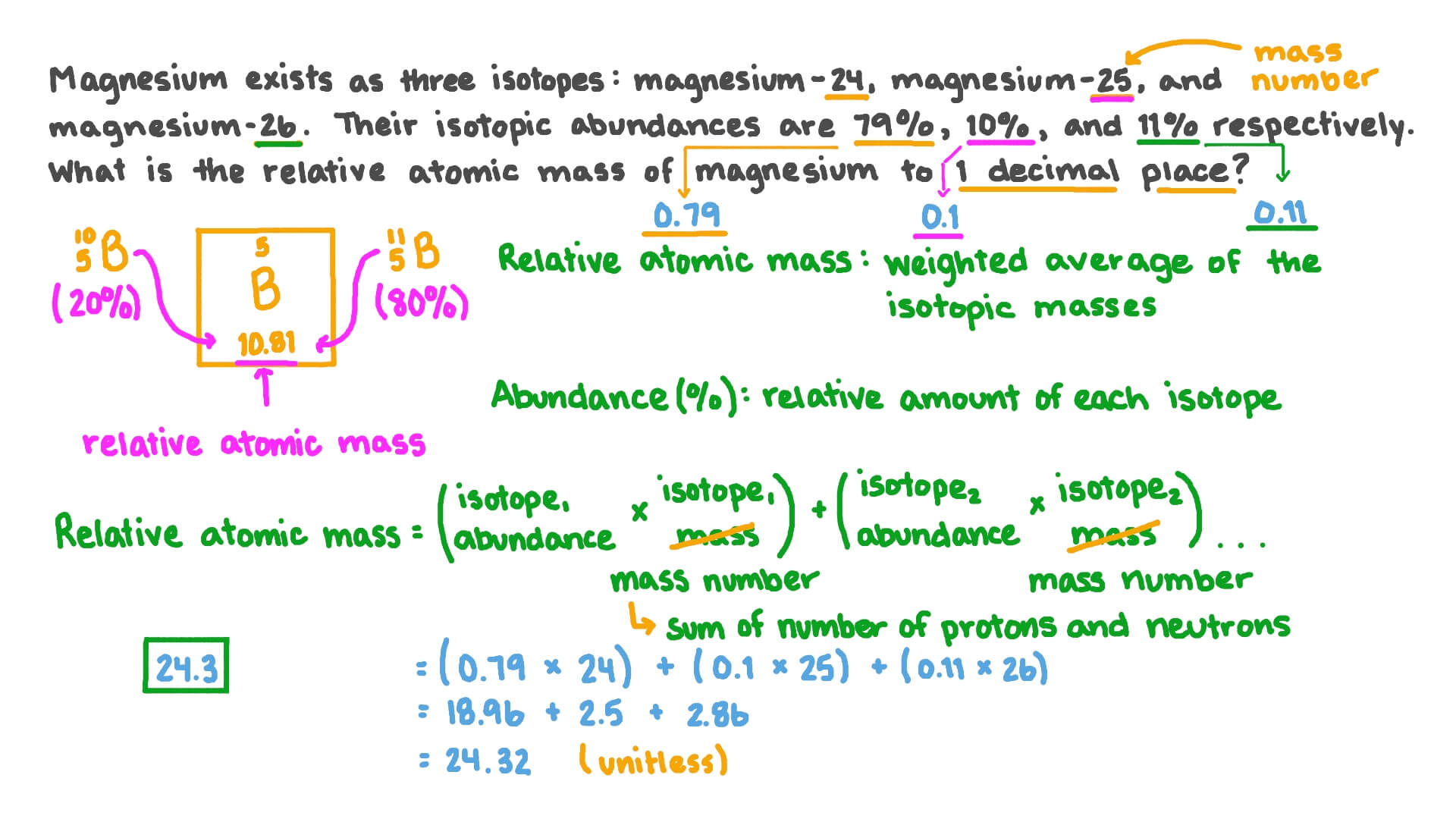
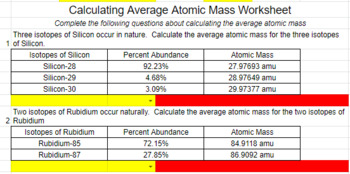
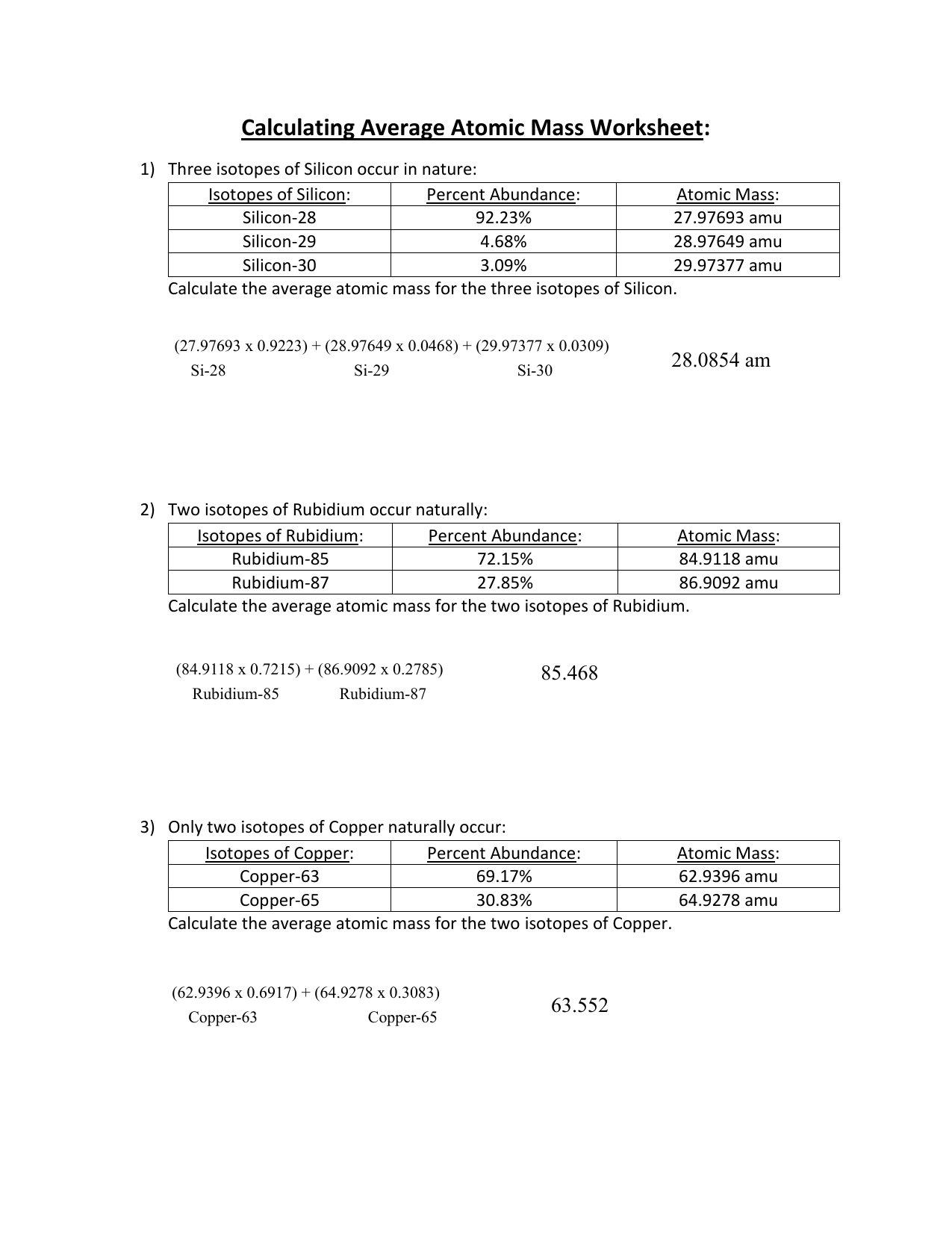
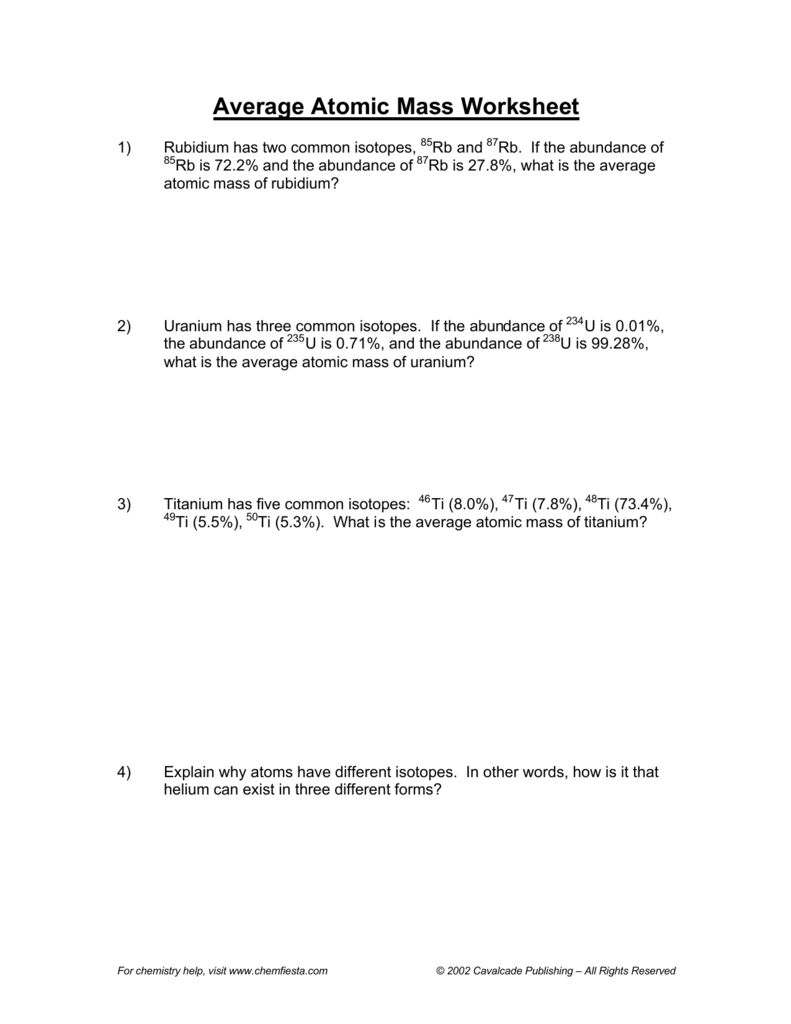
PDF Calculating Average Atomic Mass Worksheet - NMSU Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ... 43 atoms and isotopes worksheet - Worksheet Database 42 subatomic particles worksheet answers - Worksheet For You Atoms Worksheet 1 Subatomic Particles Answer Key. Some of the worksheets displayed are atomic structure work chemistry work atomic number and mass number atomic particles atoms isotopes and bonding work answer key ion symbol protons electrons charge atomic structure review work atoms and their parts subatomic particles 3 06 atomic ... › cms › lib04NAME Average Atomic Mass Worksheet: show all work. The average atomic mass of the three isotopes is 24.3050 amu. If the atomic mass of 25Mg is 24.98584 amu, and 26Mg is 25.98259 amu, calculate the actual atomic mass of 24Mg. 24Mg = 23.98504 amu 8) Complete the table Isotope Mass (amu) Relative Abundance (%) Neon-20 19.992 90.51 Neon-21 20.994 0.27 Neon-22 21.991 9.22 Fresh Isotopes And Average Atomic Mass Worksheet - The ... Showing top 8 worksheets in the category isotopes and average atomic mass. 24mg 7870 25mg 1013 and 26mg 117. Mn 42 mn 40 of protons 25 25 of neutrons 17 15 of electrons 25 25 ge 62 ge 64 of protons 32 32 of neutrons 30 32 of electrons 32 32 pd 94 pd 97 of protons. The afterward definitions may be helpful.
PDF Isotope Practice Worksheet - Chemistry 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer: 126.86 amu 10. The natural abundance for boron isotopes is 19.9% 10B and 80.1% 11B . Calculate boron's atomic mass. Answer: 126.86 amu 11. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass. Answer: 1.21 amu 12. 40 isotope worksheet answer key - Worksheet Master The numbers 12 13 and 14 refer to the Mass Number d. Isotopes And Atomic Mass Worksheet Answer Key Phet Isotopes And Atomic Mass Worksheet Answer Key Points. Gr11 Isotope Practice 1. 3 40 38 46. Carbon is composed primarily of two isotopes. Use this information to determine which isotopes of Br occur in nature. 2 180 71 109. PDF Isotopes and Average Atomic Mass This is done the same way you would find any weighted average: Calculating the Atomic Mass 1) Convert given percent abundances to decimals (divide by 100) 2) Multiply the mass of each isotope by its decimal abundance. 3) Add resulting numbers. Ex 1) Find the average atomic mass of carbon given the following: Isotope Mass (amu) Percent Abundance Isotopes Worksheet Pdf Answers - Worksheet Now Rubidium is a soft silvery white metal that has two common isotopes 85rb and 87rb. Isotopes practice worksheet. Hydrogen is 99 1h 0 8 2h and 0 2 3h. That is because the masses shown on your tables are the weighted average of all the naturally occurring isotopes. Calculate boron s atomic mass.
PDF Isotopes and Average Atomic Mass - FISD odic table reports the average atomic mass, which is a weighted average of all isotopes of boron. 8. A certain element has two isotopes. One isotope, which has a percent abundance of 72.15% has a mass of 84.9118 amu. The other isotope has a mass of 86.9092 amu. a. Calculate the average atomic mass of this element to three decimal places 84.9118 × 72.15
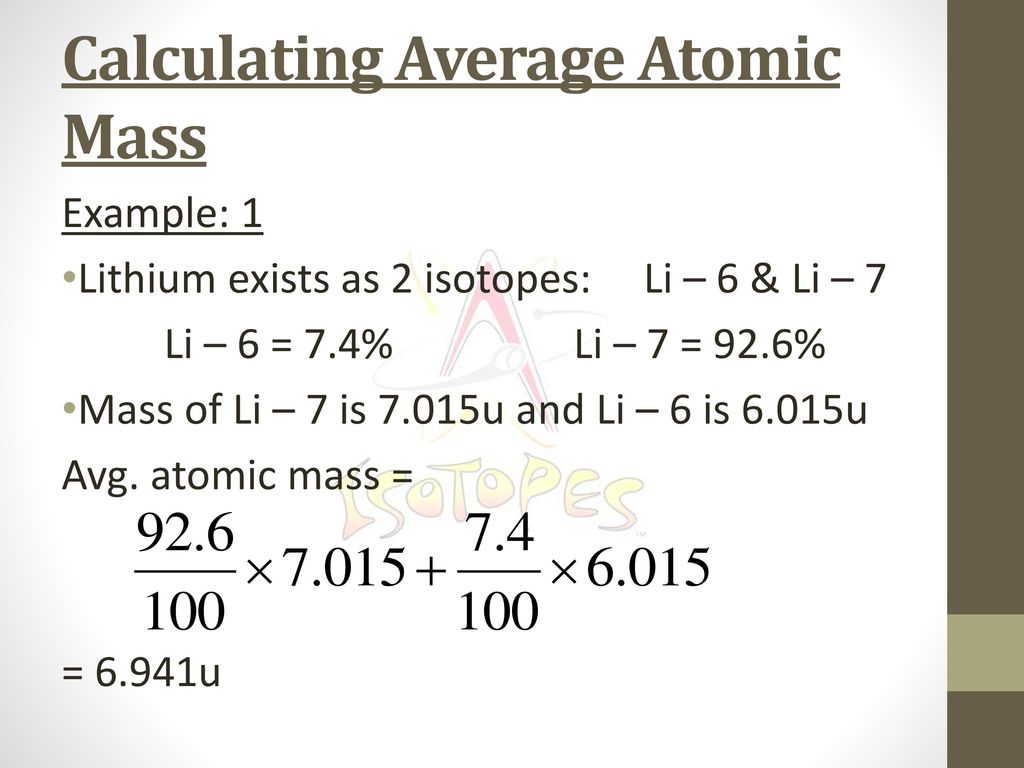
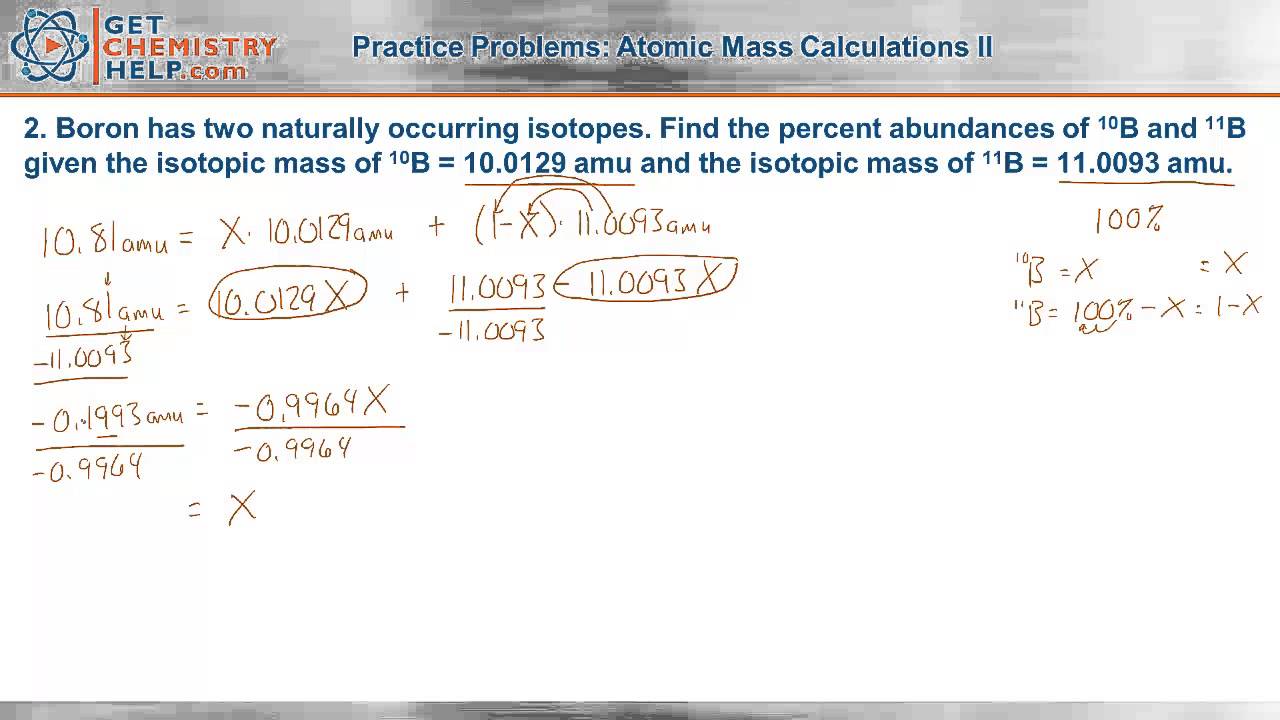
PDF Isotope Practice Worksheet Based on the atomic mass, which isotope should be more abundant? 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. 10. The natural abundance for boron isotopes is 19.9% 10B and 80.1% 11B . Calculate boron's atomic
42 average atomic mass worksheet answer key - Worksheet ... Average Atomic Mass Worksheet Answer Key Atomic Structure Worksheet. PDF Answers Key for Unit Worksheets - Livingston The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes.
39 chemistry average atomic mass worksheet answers ... DOC Chemistry Worksheet - Forestville Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0?
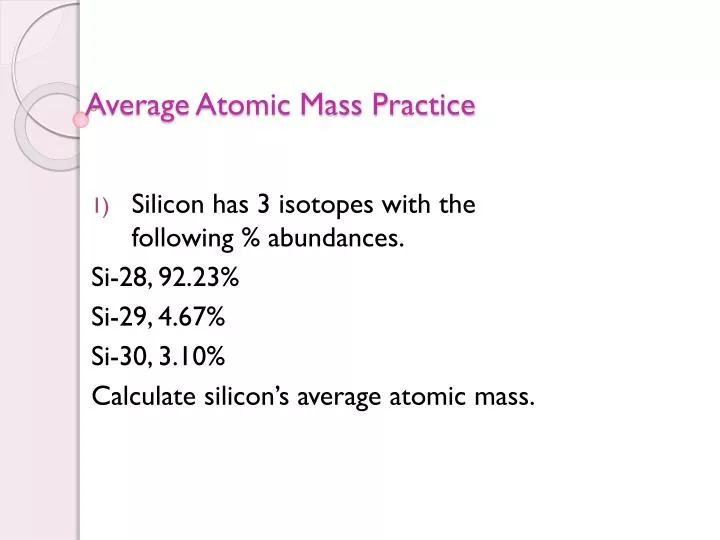
43 average atomic mass worksheet - Worksheet For You Calculate Atomic Mass Worksheet , Jobs EcityWorks Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three...
PDF KMBT 654-20131024112244 - Berger's Chemistry Class The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes. l.
› userfiles › 1012Calculating Average Atomic Mass Worksheet Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon. 2) Two isotopes of Rubidium occur naturally:
PDF Isotopes Average Atomic Mass - nyostrander.us 6. Weighted average of naturally occurring isotopes atomic mass 7. Total number of protons plus neutrons mass number 8. All electrons are in the lowest energy levels ground state 9. 1/12 the mass of a carbon-12 atom atomic mass unit (u) 10. How to solve for the number of neutrons mass number - atomic number 11.
41 calculating atomic mass worksheet answers - Worksheet ... Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. Calculating Average Atomic Mass Worksheet Name The relative abundance and atomic masses are: 69.2% for mass of 62.93u . 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. 3.
PDF Average Atomic Mass a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11.
Isotopes and Atomic mass worksheet.docx - Isotopes and ... Isotopes and Atomic mass worksheet The atomic mass of an element as stated in the periodic table is the weighted average of all of the known isotopes of that element. It is stated in atomic mass units or amu where an atomic mass unit is exactly 1/12 of the mass of carbon 12.
Isotopes And Average Atomic Mass Chemistry Worksheet ... December 21, 2021. November 29, 2021. · Chemistry Worksheet. by David G. Fisher. Isotopes And Average Atomic Mass Chemistry Worksheet Answers - Worksheets are a low-cost, high-impact source. In a number of instances, these have actually been shown to be reliable in speeding up trainee learning. It is among the most commonly made use of ...
PDF Atoms and Isotopes Worksheet - Tumwater School District Isotope Isotope Notation Atomic # Protons Electrons Neutrons Nickel-58 15 15 53 74 36 48 34 45 Calcium-40 Chlorine-37 9. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Show all work. Mass of Isotope % abundance 36.96590 24.47 34.96885 75.53
PDF Answers Key for Unit Worksheets - Livingston The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
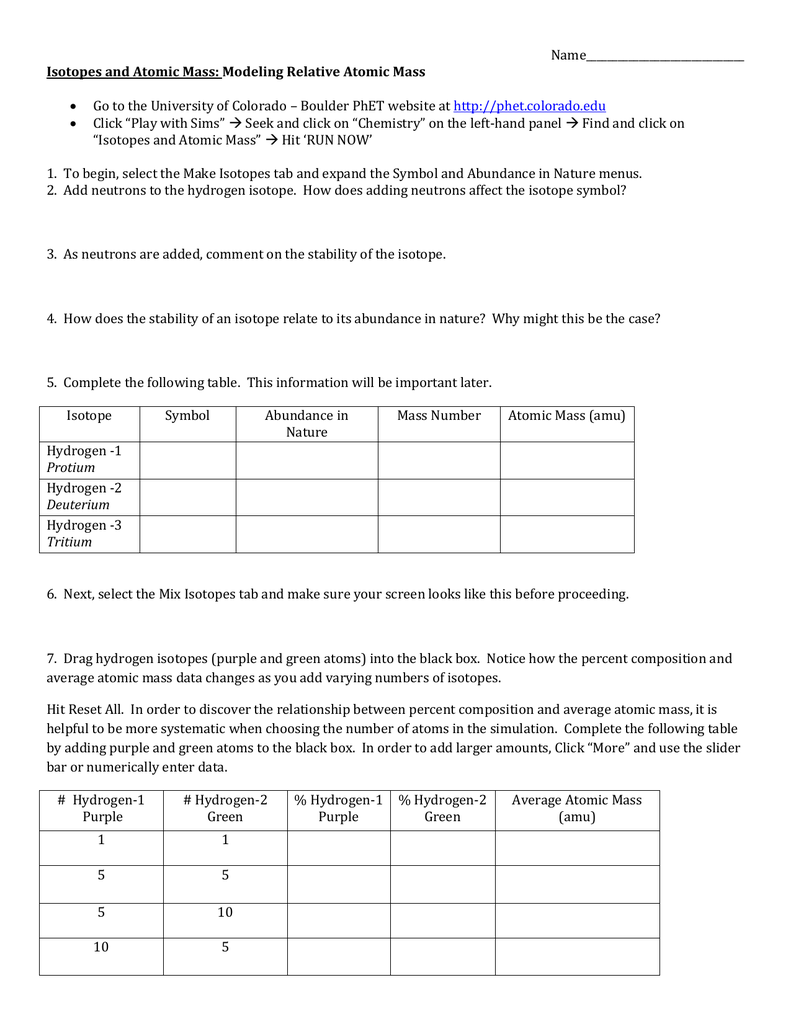
phet.colorado.edu › isotopes-and-atomic-massIsotopes and Atomic Mass - Isotopes | Atomic Mass - PhET ... Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
courses.lumenlearning.com › cheminter › chapterCalculating Atomic Mass | Chemistry for Non-Majors Sample Problem: Calculating Atomic Mass . Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine. Step 1: List the known and unknown quantities and plan the problem. Known . chlorine-35: atomic mass = 34.969 amu and % abundance = 75.77%
Isotopes And Average Atomic Mass Chemistry Worksheet ... Isotopes And Average Atomic Mass Chemistry Worksheet - Worksheets are an inexpensive, high-impact source. In several circumstances, these have actually been revealed to be effective in quickening student discovering. It is among the most extensively used training help worldwide today, as well as for good factor.
PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14.






















0 Response to "40 isotopes and average atomic mass worksheet"
Post a Comment