41 thermochemistry problems worksheet number one answers
thermochemistry questions and answers - Free Textbook PDF Thermochemistry Problems - Worksheet Number One (answers available on web site). 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0. °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. How much ... WS1-Thermochem.pdf Thermochemistry Worksheet Answers - worksheet Thermochemistry worksheet answers. 390 0 01 390 c 3. A series of free high school chemistry video lessons. Q 20 0 g 20 0 c 2 02 j g c. Displaying top 8 worksheets found for thermochemistry with answers. We will ignore any heats losses to the walls of the container and losses to the air. Thermochemistry answers worksheet number one.
PDF (Cp 0c) - srvhs.org Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3.
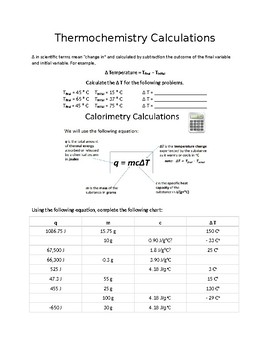
Thermochemistry problems worksheet number one answers
Heat Problems Specific Answers Worksheet [ZG8BKM] 6 g 120 g (rounded answer for sig. How much heat in. 75-g piece of iron sorbs 1086. Specific Heat Problems. Calorimetry practice worksheet answers Heat calorimetry problems show your work box your answers equations. Thermochemistry Answers - Worksheet Number One. 0 °C to 303. The addition of 3. 7+ Fresh Thermochemistry Worksheet With Answers - Markdrum ... THERMOCHEMISTRY CALCULATIONS WORKSHEET 1. Answers are given on the last pages. Do the questions and follow along with this video for when you get stuck. 2 - Chemical Equilibrium Worksheet 2-1 - Equilibrium Enthalpy and Entropy Page 3 17. Heat and qmcΔT Questions 1-5. The reaction of magnesium with sulfuric acid was carried out in a calorimeter. Thermochem WS #1 Answers - ChemTeam Thermochemistry Answers - Worksheet Number One. We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C). (Note C p of gas is used.)
Thermochemistry problems worksheet number one answers. Problems I - Thermochemistry Problems - Worksheet Number ... Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. Thermochemistry Practice Problems Worksheet Answers ... Download file pdf thermochemistry worksheet 1 answers thermochemistry problems worksheet number one thermochemistry exam1 and problem solutions. We will ignore any heats losses to the walls of the container and losses to the air. Na 2 o s so. Calculate the heat of the reaction for the following reaction. 6+ Fresh Thermochemistry Calculations Worksheet Answers ... Thermochemistry answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. Thermochemistry problems worksheet number one answers. G x 2260 j g 2 260 000 j q m x c x δt. Is this reaction exothermic or endothermic. Displaying top 8 worksheets found for thermochemistry with answers. PDF Thermochemistry Problems - Laney College [11] For this problem, we do not know the temperature change that either the brick or the water is undergoing. We do know that q water = -q bricks. First, find the heat capacity, not the specific heat of 1000 gal of water. Then, use that number to find the number of bricks. C = 1.0 X 103 gal X 4 qt 1 gal X 1 L 1.0567 qt X 1000 mL L X 1.00 g 1 ...
PDF 21. The graph below shows a pure substance which is heated ... Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. PDF Thermochemistry Problems Worksheet Number One Answers thermochemistry problems worksheet number one answers download test bank for chemistry the central science 12th. cie 9701 gce chemistry past papers past papers as a2 a. chemistry with lab - easy peasy all in one high school. printable crossword puzzles. courses a to z index golden west college. port manteaux word maker onelook dictionary ... 43 thermochemistry problems worksheet number one answers ... Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. DOC Thermochemistry Answers - Worksheet Number One Thermochemistry Answers - Worksheet Number One We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 ツーC) (2.02 J/g ツーC)= 808 J.
PDF Student Worksheet for Thermochemistry An unknown substance with an unknown mass absorbs 1500J while undergoing a temperature increase of 30°C. It's specific heat is 0.49 J/g°C. What is the mass of the substance? 3. If the temperature of 50.1 g of ethanol increases from 35°C to 87.8°C, how much heat has been absorbed by the ethanol? The specific heat of ethanol is 2.44J/g°C. 4. PDF Thermochemistry Example Problems 1 Thermochemistry Example Problems Recognizing Endothermic & Exothermic Processes On a sunny winter day, the snow on a rooftop begins to melt. As the melted water drips from the roof, it refreezes into icicles. Describe the direction of heat flow as the water freezes. Is this process endothermic or exothermic? Thermochemistry And Thermodynamics Worksheet 2 Answers ... Thermochemistry and thermodynamics worksheet 2 answers. Thermochemistry answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. 2F 2 g 2H 2 Ol 4HFg O 2 g H. Entropy and the 2nd and 3rd Laws of Thermodynamics. 2f 2 g 2h 2 o l 4hf g o 2 g h. 5+ Popular Thermochemistry Worksheet With Answers ... Answers to Thermochemistry Answers to Thermochemistry Worksheets Heat Worksheet 1. The reaction of magnesium with sulfuric acid was carried out in a calorimeter. 245 Jg⁰C 508 g 8. 2 2 267g 078345 mol H S 3408 gmol H S so 406 kJ was released when 078345 moles of H 2 S reacted. 3 Describe what we mean by. Free Thermochemistry Worksheets.
PDF Answers, Thermochemistry Practice Problems 2 Answers, Thermochemistry Practice Problems 2 1 6. When 26.7 g of H 2 S was burned in excess oxygen, 406 kJ was released. What is H for the following equation? 2 H 2 S(g) + 3 O 2 (g) 2 SO 2 (g) + 2 H 2 O(g); H = ??? Answer: -1040 kJ Reasoning: 2 2 26.7g 0.78345 mol H S 34.08 g/mol H S, so 406 kJ was released when 0.78345 moles of H 2 S reacted. 2 2
PDF Practice Thermochemistry Problems With Answers Thermochemistry Problems Worksheet Number One. AP Chemistry Thermochemistry Lecture Outline. Enthalpy Problems And Answers Bing. 10 E ... DECEMBER 13TH, 2019 - ANSWERS THERMOCHEMISTRY PRACTICE PROBLEMS 2 1 6 WHEN 26 7 G OF H 2 S WAS BURNED IN EXCESS OXYGEN 406 KJ WAS RELEASED WHAT IS H FOR THE FOLLOWING'
Thermochemistry Worksheet With Answers - Weavingaweb Answers, thermochemistry practice problems 2 1 6. 1) what is happening to the average potential energy of the molecules in the sample during section 3? 2Nh 3 22Kcal N 2 3H 2 H 2. We will ignore any heats losses to the walls of the container and losses to the air. The reaction of magnesium with sulfuric acid was carried out in a calorimeter.
Of The Best Thermochemistry Worksheet With Answers - Labelco Thermochemistry With Answers - Displaying top 8 worksheets found for this concept. Based on the enthalpy change determine whether the reaction is endothermic or exothermic and whether it would be thermodynamically favored or unfavored. When matters change state from liquid to gas they absorb energy. 2nh 3 22kcal n 2 3h 2 h 2.
Thermochem WS #1 Answers - ChemTeam Thermochemistry Answers - Worksheet Number One. We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C). (Note C p of gas is used.)
7+ Fresh Thermochemistry Worksheet With Answers - Markdrum ... THERMOCHEMISTRY CALCULATIONS WORKSHEET 1. Answers are given on the last pages. Do the questions and follow along with this video for when you get stuck. 2 - Chemical Equilibrium Worksheet 2-1 - Equilibrium Enthalpy and Entropy Page 3 17. Heat and qmcΔT Questions 1-5. The reaction of magnesium with sulfuric acid was carried out in a calorimeter.
Heat Problems Specific Answers Worksheet [ZG8BKM] 6 g 120 g (rounded answer for sig. How much heat in. 75-g piece of iron sorbs 1086. Specific Heat Problems. Calorimetry practice worksheet answers Heat calorimetry problems show your work box your answers equations. Thermochemistry Answers - Worksheet Number One. 0 °C to 303. The addition of 3.






![Grade 10 Chemistry: Hess's Law] Does anyone know how to solve ...](https://preview.redd.it/xaqarzdo4d251.jpg?auto=webp&s=c49670fd3759a50c5ad16ba02ede7f4e1dcda3cb)






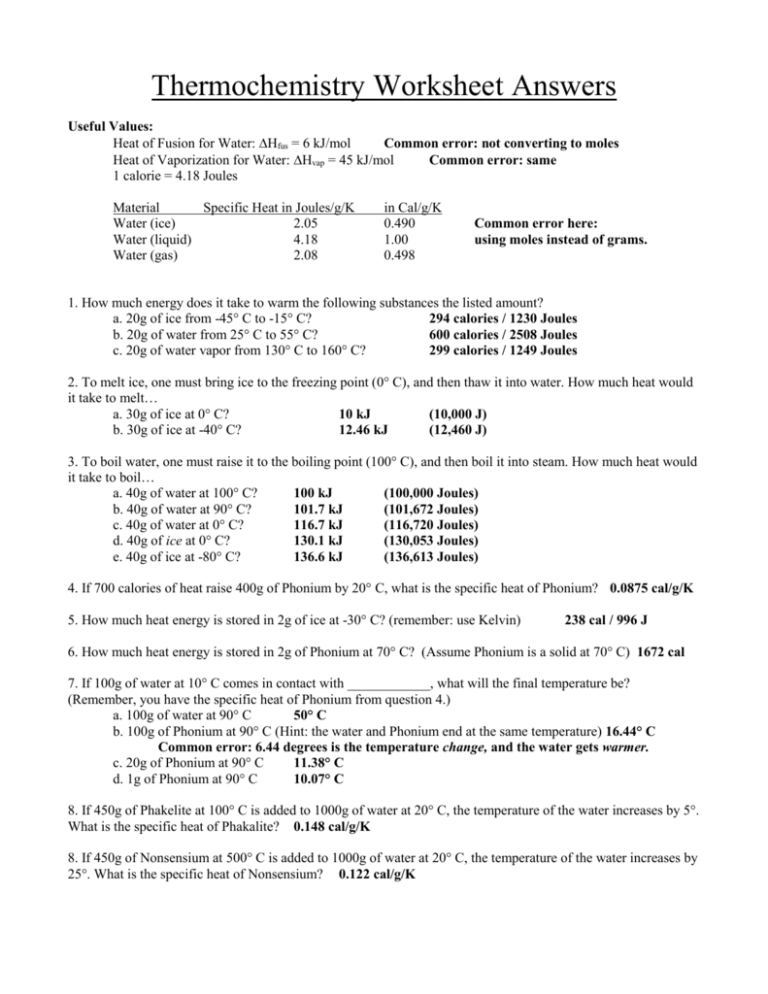
![document - [PDF Document]](https://cdn.vdocuments.mx/img/1200x630/reader024/reader/2021010615/5866deb91a28abd7408b93dc/r-1.jpg?t=1645744265)












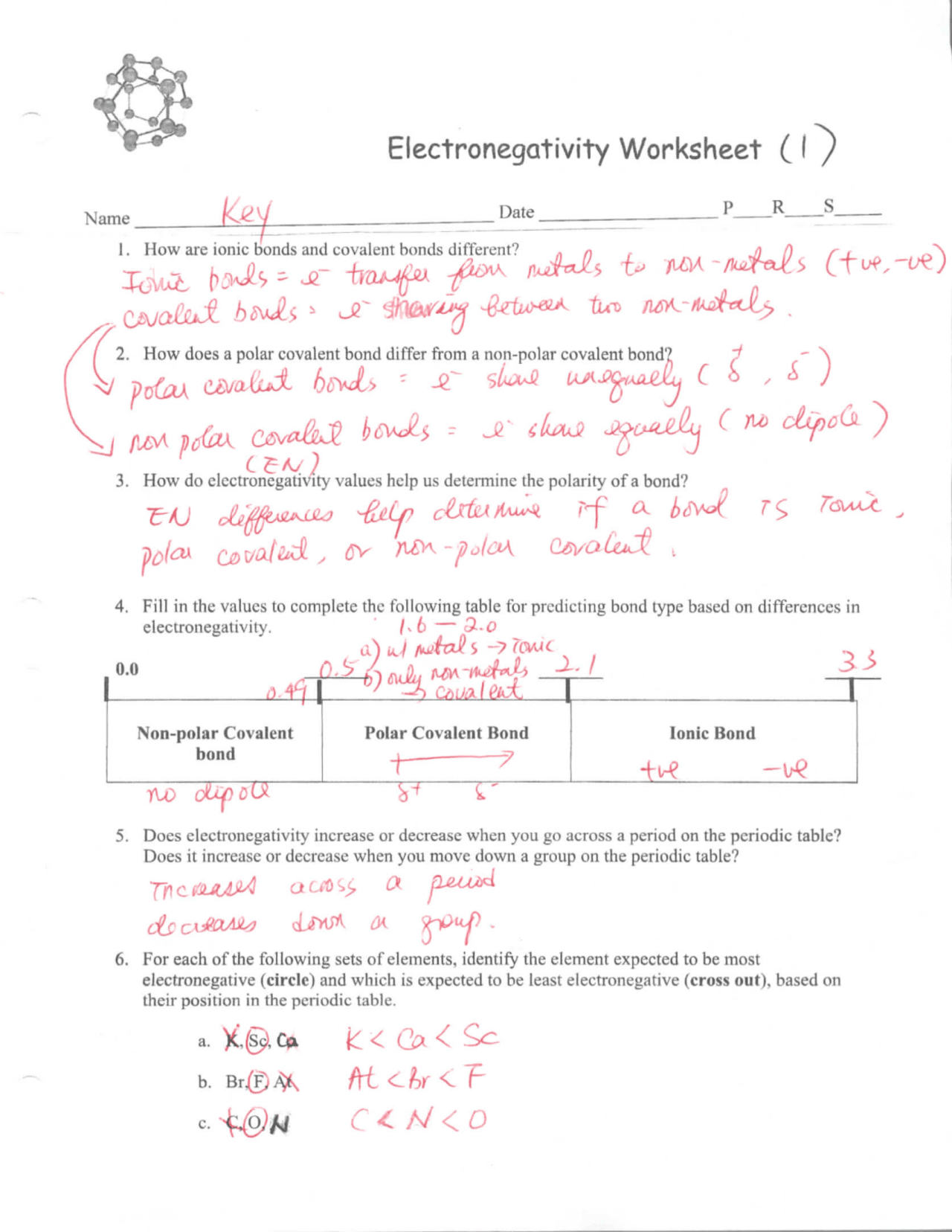

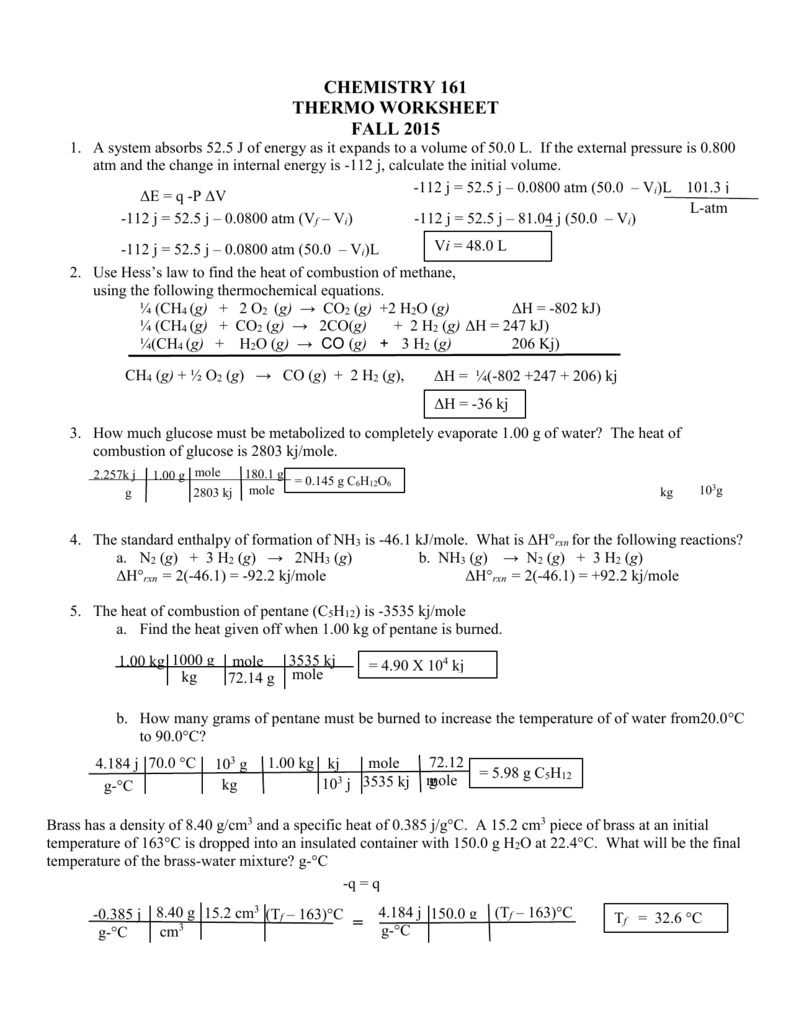

0 Response to "41 thermochemistry problems worksheet number one answers"
Post a Comment