42 the mole and avogadro's number worksheet
The Mole and Avogadro's Number - Flagstaff Arts and ... mole of water molecules is 6.022 X 1023 water molecules. The NIST 2007 value of Avogadro's number is 6.022 141 79 ± 0.000 000 30 X 1023 mol–1. For most calculations, a rounded value of 6.022 X 1023 (four significant figures) is satisfactory. This is an incredibly large number. A mole of say, grapefruit, stacked together, would occupy the Freezing and Boiling Points - CliffsNotes That concentration is the number of moles per kilogram of benzene, but the solution used only 300 grams of the solvent. The moles of santonic acid is found as follows: 0.3 kg × 0.676 mole/kg = 0.203 mole. and the molecular weight is calculated as
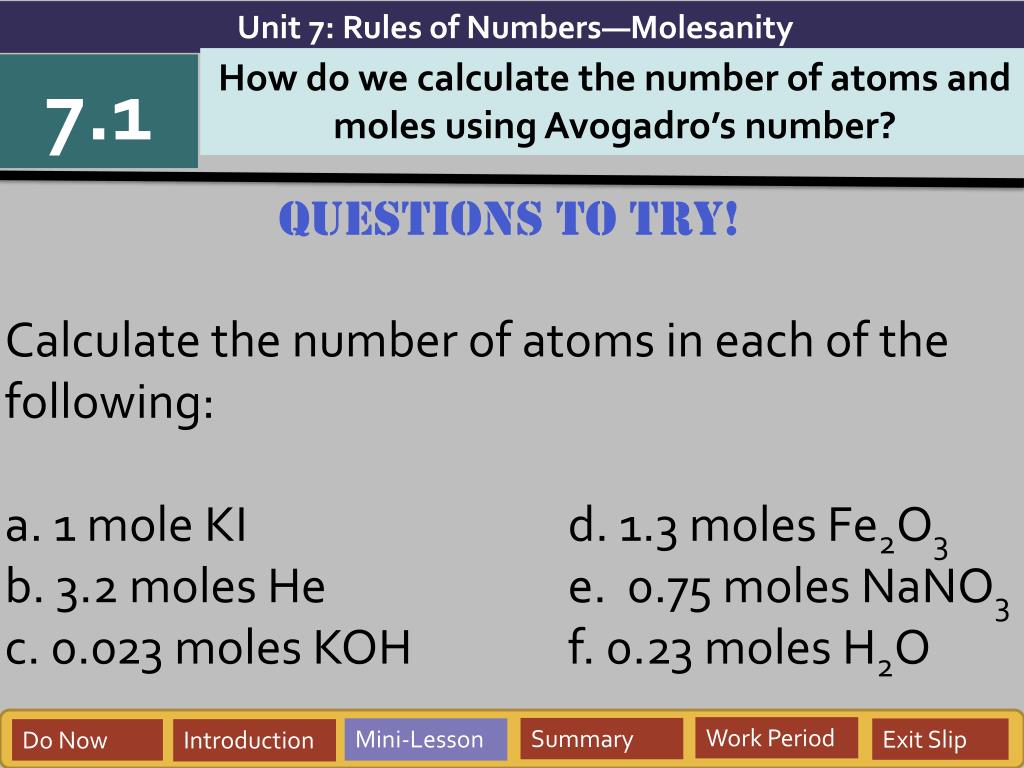
Skills Worksheet Problem Solving The number 6.022 137 1023is called Avogadro’s number. For most purposes it is rounded off to 6.022 1023. Because this is an awkward number to write over and over again, chemists refer to it as a mole(abbreviated mol). 6.022 1023objects is called a mole, just as you call 12 objects a dozen.

The mole and avogadro's number worksheet
20 Avogadro Number Worksheet Answers | Worksheet From Home 32 The Mole And Avogadros Number Worksheet Answers. 32 The Mole And Avogadros Number Worksheet Answers via : isme-special.blogspot.com. Avogadro s Number Using the Mole to Count Atoms Video. Avogadro s Number Using the Mole to Count Atoms Video via : study.com. 17 2 The Avogadro Number PDF Free Download Chemistry And Avogadros Number Worksheets - K12 Workbook Chemistry And Avogadros Number. Displaying all worksheets related to - Chemistry And Avogadros Number. Worksheets are The mole and avogadros number, Example exercise atomic mass and avogadros number, Avogadros number problems work, Avogadros number practice work, Skills work problem solving, The mole chemistry lesson plan overview day 11, Chemistry mole to mole conversions work, H2fromh2o lesson plans and work updated thursday. Mastering Biology: Chapter 4 Flashcards & Practice Test ... 16.9.2013 · A mole (abbreviated mol) is defined as an exact number of objects: 6.02 × 1023 (Avogadro's number). You can have 1 mole of an atom, a molecule, or any other object, such as eggs or books. One mole of an object equals 6.02 × 1023 of that object. The table shows the "molar ratios" of some of the products from the Miller H2S experiment.
The mole and avogadro's number worksheet. Molar Mass | Boundless Chemistry - Lumen Learning One mole (abbreviated mol) is equal to the number of atoms in 12 grams of carbon-12; this number is referred to as Avogadro’s number and has been measured as approximately 6.022 x 10 23. In other words, a mole is the amount of substance that contains as many entities (atoms, or other particles) as there are atoms in 12 grams of pure carbon-12. Mole-to-Mole Ratios and Calculations of a Chemical ... 17.11.2021 · A mole is a chemical counting unit, such that 1 mole = 6.022*10 23 particles. Stoichiometry also requires the use of balanced equations. From the balanced equation we can get the mole ratio. How to Calculate Mass Percent Composition - ThoughtCo 24.11.2019 · Step 2: Find the number of grams of each component make up one mole of CO 2. One mole of CO 2 contains 1 mole of carbon atoms and 2 … chemistry worksheet molar masses and avogadro's number Solve the following problems involving the mole concept. Problems 1-2: moles to grams AND grams to moles. 1. How many grams are there in 11.8 moles of sodium ...
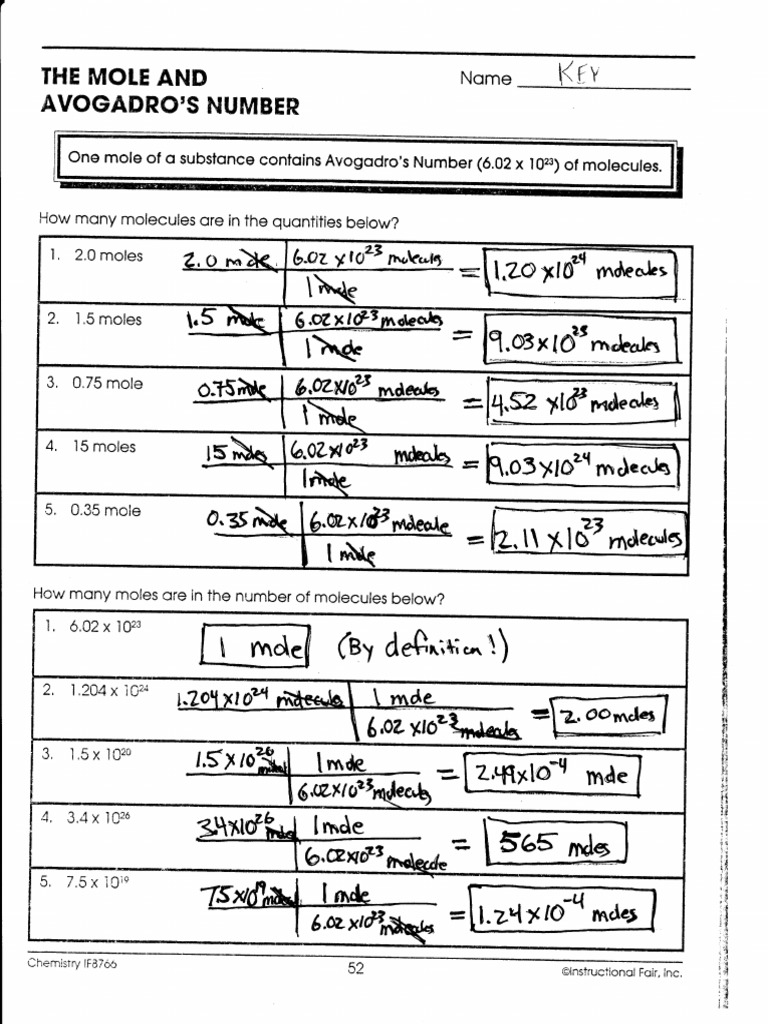
Of The Best The Mole And Avogadro Number Worksheet Answers ... The mole and avogadros number worksheet answers 602 x 10 23 individual atomsa value called avogadros number after the chemist amedeo avogadro. 050 moles of 170 moles of KMn04 025 moles of KCI Ecl. Explore fun printable activities for K-8 students covering math ELA science more. Avogadros number is the number of particles in a mole 602 10 23. Mole and Avogadro's Number Worksheet with Answers ... 20 Apr 2021 — Avogadro's number contain 6.02 x 1023 molecules, and mole is the amount of substance containing the same number of discrete entities which ... Stoichiometry questions (practice) - Khan Academy The mole and Avogadro's number. Stoichiometry example problem 1. Stoichiometry. Limiting reactant example problem 1 edited. Specific gravity. Next lesson. Balancing chemical equations. Stoichiometry article. Up Next. Stoichiometry article. Our mission is to provide a free, world-class education to anyone, anywhere. The Mole and Avogadro’s Number One mole of a substance contains Avogadro’s Number (6.02 x1023) of molecules. Directions: How many molecules are in the quantities below? 1) 3.0 moles ANSWER: 1.8 E 24 molecules 2) 2.75 moles ANSWER: 1.66 E 24 molecules 3) 0.82 moles ANSWER: 4.8 E 23 molecules 4) 12 moles ANSWER: 7.2 E 24 5) 0.74 moles ANSWER: 4.5 E 23 molecules
Student Worksheet for 1-D Kinematics - Science Learning Space Avogadro’s number, 23 -1 N 0 6.02 10 mol Universal gas constant, R 8.31 J (mol K) Boltzmann’s constant, 1.38 10 J K23 k B Electron charge magnitude, e 1.60 10 C 19 1 electron volt, 1 eV 1.60 10 J Speed of light, c 3.00 10 m s8 Universal gravitational constant, G 6.67 10 m kg s11 3 2 Acceleration due to gravity at Earth’s surface, g 9.8 m s2 Gas Laws: Boyle's Law, Charle's Law, Gay-Lussac's Law ... The number of molecules in a mole of any gas is known as the Avogadro’s constant and is calculated to be 6.022 * 10 23. The values for temperature and pressure here are the standard values. For temperature, we take it to be 273.15 K while for … CHEMISTRY WORKSHEET # 2 MOLE PROBLEMS—THE MOLE AS … CHEMISTRY WORKSHEET # 3 AVOGADRO’S NUMBER. One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a . mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms. Mastering Biology: Chapter 4 Flashcards & Practice Test ... 16.9.2013 · A mole (abbreviated mol) is defined as an exact number of objects: 6.02 × 1023 (Avogadro's number). You can have 1 mole of an atom, a molecule, or any other object, such as eggs or books. One mole of an object equals 6.02 × 1023 of that object. The table shows the "molar ratios" of some of the products from the Miller H2S experiment.
Chemistry And Avogadros Number Worksheets - K12 Workbook Chemistry And Avogadros Number. Displaying all worksheets related to - Chemistry And Avogadros Number. Worksheets are The mole and avogadros number, Example exercise atomic mass and avogadros number, Avogadros number problems work, Avogadros number practice work, Skills work problem solving, The mole chemistry lesson plan overview day 11, Chemistry mole to mole conversions work, H2fromh2o lesson plans and work updated thursday.
20 Avogadro Number Worksheet Answers | Worksheet From Home 32 The Mole And Avogadros Number Worksheet Answers. 32 The Mole And Avogadros Number Worksheet Answers via : isme-special.blogspot.com. Avogadro s Number Using the Mole to Count Atoms Video. Avogadro s Number Using the Mole to Count Atoms Video via : study.com. 17 2 The Avogadro Number PDF Free Download

The mole and Avogadro’s number | Chemistry worksheets, Electron configuration, High school chemistry







0 Response to "42 the mole and avogadro's number worksheet"
Post a Comment