40 ph and poh worksheet
› gchelp › howtosolveitCalculating_pHandpOH - Purdue University The relationship between pH and pOH; Calculating pK a; Calculating K a from pK a; Calculating pK b; Calculating K b from pK b; Calculating pH. To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter . The pH is then calculated using the expression: pH = - log [H 3 O +]. Acids/Bases & pH Worksheet - St. Louis Public Schools Acids/Bases & pH Worksheet (continued) Complete the following table by filling in the empty spaces. Indicate if the solution is acidic, basic or neutral. You should be able to do the starred (*) item s without a calculator. (Problem #23-34) [H 3O] …
› w335-ph-worksheet-3Calculating pH and pOH worksheet - Everett Community College Calculating pH and pOH worksheet W 335 Everett Community College Tutoring Center Student Support Services Program 1) What is the pH of a 0.0235 M HCl solution? ... The concentrations of H+ and OH-are equal, as are the pH and pOH, so the solution must be neutral. Title: Calculating pH and pOH worksheet Author: Moira O'Toole
Ph and poh worksheet
Calculating pH and pOH worksheet - Everett Community … Calculating pH and pOH worksheet W 335 Everett Community College Tutoring Center Student Support Services Program 1) What is the pH of a 0.0235 M HCl solution? ... The concentrations of H+ and OH-are equal, as are the pH and pOH, so the solution must be neutral. Title: Calculating pH and pOH worksheet Author: Moira O'Toole PDF pH, pOH, Ka pKa worksheet - Mr. Bigler pH, pOH, K a & pK a worksheet Calculate the pH of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. 1. ... 7. pOH = 9.39 8. pH = 2.54. 9. An 0.100M solution of nitrous acid (HNO 2) has a pH of 2.17. (a) What is [H+] for this solution? PDF Ph And Poh Worksheet With Answers Get Free Ph And Poh Worksheet With Answers Ph And Poh Worksheet With Answers This is likewise one of the factors by obtaining the soft documents of this ph and poh worksheet with answers by online. You might not require more epoch to spend to go to the ebook opening as well as search for them. In some cases, you likewise accomplish not discover the
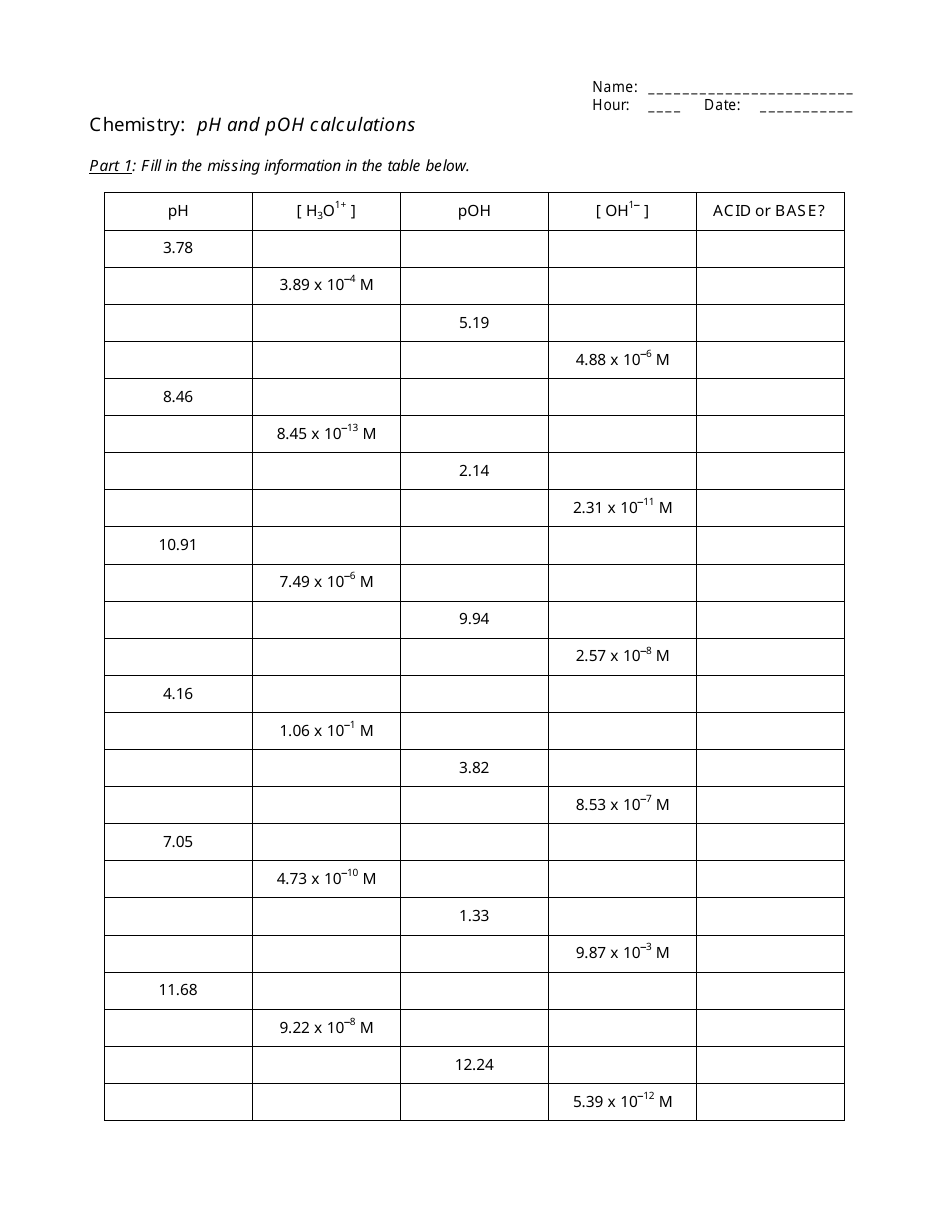
Ph and poh worksheet. PDF Ph And Poh Continued Worksheet Answers could enjoy now is ph and poh continued worksheet answers below. Copy Code www Page 1/1. Title: Ph And Poh Continued Worksheet Answers Author: Subject: Ph And Poh Continued Worksheet Answers Keywords: ph, and, poh, continued, worksheet, answers DOC pH & pOH - Central Bucks School District B. If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution? KEY. Chemistry: pH and pOH calculations. Part 1: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 ... PDF pH and pOH Calculations - Just Only pH and pOH Calculations 1) Determine the pH of a 0.00340 M HNO3 solution. 2) Determine the pOH of a 0.00340 M HNO3 solution. 3) Determine the pH of a 4.30 x 10-4 M NaOH solution. 4) If a solution is created by adding water to 2.30 x 10-4 moles of NaOH and 4.50 x 10-6 moles of HBr until the final volume is 1.00 L, what is the pH of this solution? › cms › libAcids/Bases & pH Worksheet - St. Louis Public Schools Acids/Bases & pH Worksheet (continued) Complete the following table by filling in the empty spaces. Indicate if the solution is acidic, basic or neutral. You should be able to do the starred (*) item s without a calculator. (Problem #23-34) [H 3O] + [OH]− pH pOH Acidic/Basic/Neutral 2.35 × 10−3. 4.93 ×
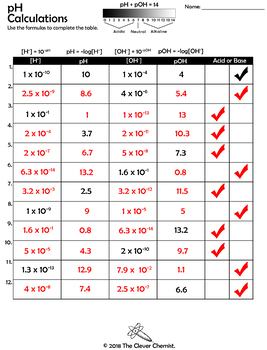
The pH Scale: Calculating the pH of a Solution - Study.com Aug 24, 2021 · In chemistry, the pH scale is used to measure acidity on a scale of 0-6 for acids, 7 for neutral, and 8-14 to represent basic. Take an in-depth look into calculating the pH of … Calculating_pHandpOH - Purdue University The pH and pOH of a water solution at 25 o C are related by the following equation. pH + pOH = 14 If either the pH or the pOH of a solution is known, the other can be quickly calculated. Example: A solution has a pOH of 11.76. What is the pH of this solution? pH = 14 - pOH = 14 - … pH and pOH Practice Worksheet - ThoughtCo Nov 30, 2018 · Todd Helmenstine. This downloadable PDF worksheet is for students to practice calculating pH and pOH values from concentration values of H + and OH-ions.. Useful relationships: pH = -log[H +] pOH = -log[OH-] k water = 1 x 10-14 = [H +][OH-] pH + pOH = 14 Review: pH Calculations: Chemistry Quick Review of pH DOC pH and pOH Calculations Worksheet pH and pOH Calculations Worksheet #2. Find the pH and the pOH of the solution. Show your work and use correct sig. figs. pH pOH. 1. A 0.023 M solution of hydrochloric acid. 2. A 6.6 x 10 -6 M solution of nitric acid. 3. A 0.0334 M solution of potassium hydroxide. 4. A 1.0 x 10 -5 M solution of hydroarsenic acid. 5.
PDF Worksheet: pH and pOH Worksheet: pH and pOH 1. -If the hydrogen ion concentration of a solution is 1.30 x 10 4 M a. What is the pH of the solution? b. What is the pOH of the same solution? c. What is the hydroxide ion concentration of the solution? 2. If the hydroxide ion concentration of a solution is 2.8 x 10-6 M a. Is it an acidic or basic solution? b. Acid And Bases Ph Calculations Worksheets - K12 Workbook Worksheets are Acid and base ph calculations supplemental work key, Acidsbases ph work, Work 21, Calculating ph and poh work, Test2 ch17a acid base practice problems, Acid base equilibria and calculations, Acid base equilibria and calculations, Acid base calculations with salts. *Click on Open button to open and print to worksheet. › calculating-ph-and-pohCalculating pH and pOH - High School Chemistry - Varsity Tutors Possible Answers: Correct answer: Explanation: pH and pOH are the log concentrations of protons and hydroxide ions, respectively. The sum of pH and pOH is always 14. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant for the ionization of water, which is equal to . PDF Acid-Base Strength, K , pH, pOH and % Dissociation Worksheet , pH, pOH and % Dissociation - Worksheet Use the Table of Relative Strengths of Acids and Bases (Appendix B in MHR or appendix C9 in Nelson) to answer the following questions/problems:
ph and pOH worksheet.doc - Google Docs ph and pOH worksheet.doc - Google Docs. Name: Date: Hr: pH and pOH Practice Sheet 1. 1) What is the pH of a 0.0235 M HCl solution? 2) What is the pOH of a 0.0235 M HCl solution? 3) What is the pH of a 6.50 x 10-3 M KOH solution? (Hint: this is a basic solution - concentration is of OH -) 4) What is the pH of a 6.2 x 10-5 M NaOH solution?
› userfiles › 2505pH pOH [H [OH pH WORKSHEET pH—pOH—[H+]—[OH-] 1. Calculate the values of both pH and pOH of the following solutions: pH pOH a. 0.020 M HCl b. 0.0050 M NaOH c. A blood sample 7.2 x 10-8M of H+ d. 0.00035 M KOH 2. Find the values of [H+], pOH, [OH-], that correspond to each of the following pH values: [H+] [OH-] pOH a. pH of lemon juice = 2.90
› ph-and-poh-practice-worksheetpH and pOH Practice Worksheet - ThoughtCo Nov 30, 2018 · This downloadable PDF worksheet is for students to practice calculating pH and pOH values from concentration values of H + and OH-ions. Useful relationships: pH = -log[H +] pOH = -log[OH-] k water = 1 x 10-14 = [H +][OH-] pH + pOH = 14 Review: pH Calculations: Chemistry Quick Review of pH
PDF Calculating the [H3O ], [OH ], pH and pOH in Aqueous Solutions: Worksheet Calculating the [H 3 O+], [OH-], pH and pOH in Aqueous Solutions: Worksheet 1. The concentration of hydroxide ions, OH-(aq), in a solution at 25°C is 0.150 mol/L. Determine the concentration of hydronium ions, H 3 O+(aq), in the solution. 2. A solution of lithium hydroxide, LiOH(aq), is made by placing 2.00 mol of the base into 1.50 L of
Test2 ch17a Acid-Base Practice Problems pH Calculations; Relationships between pH and pOH p4 Answers p12 K a: Sense + Calculations. Using K a or pK a to Calculate [H+] and/or pH; using pH to calculate K a or pK a p5 Conceptual Questions. Acids, Bases, and Conjugates, Miscellaneous 1. In the Brønsted–Lowry definition of acids and bases, an acid _____ a. is a proton donor. ...
DOC pH & pOH B. If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution? KEY. Chemistry: pH and pOH calculations. Part 1: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 ...
Calculating pH and pOH - High School Chemistry - Varsity Tutors Since we have the concentration of hydroxide ions, we can solve for the pOH of the solution. The question asks us to find the pH of the solution, so we will need to convert pOH to pH. To do so, we simply subtract the pOH from 14. The pH of the solution is 12.3. Because sodium hydroxide is a strong base, it makes sense that the pH is above 7.
pH and pOH Part 2: For each of the problems below, assume 100% dissociation. 1. A. Write the equation for the dissociation of hydrochloric acid. B. Find the pH …
pH pOH [H [OH pH WORKSHEET pH—pOH—[H+]—[OH-] 1. Calculate the values of both pH and pOH of the following solutions: pH pOH a. 0.020 M HCl b. 0.0050 M NaOH c. A blood sample 7.2 x 10-8M of H+ d. 0.00035 M KOH 2. Find the values of [H+], pOH, [OH-], …
teachnlearnchem.com › Keys Worksheets › Acid KeypH and pOH Part 2: For each of the problems below, assume 100% dissociation. 1. A. Write the equation for the dissociation of hydrochloric acid. B. Find the pH of a 0.00476 M hydrochloric acid solution. 2. A.
DOCX pH and pOH - DeKalb County School District Author: John Bergmann and Jeff Christopherson Created Date: 02/28/2017 03:15:00 Title: pH and pOH Subject: Chemistry Keywords: pH, pOH, acid, base Category
PH and pOH worksheet - Liveworksheets.com ID: 1539333 Language: English School subject: Chemistry Grade/level: 12 Age: 16+ Main content: Acid-Base Equilibrium Other contents: pH and pOH Add to my workbooks (16) Download file pdf Embed in my website or blog Add to Google Classroom
pH_practice_worksheet.docx - pH practice Worksheet 1) Find the pH and ... Unformatted text preview: pH practice Worksheet 1) Find the pH and pOh if [H+] = 5 M. 2) Find the pH and pOh if [OH-] = 0.04 M 3) Find the pH and pOH if [H+] = 0.007 M 4) How many times more concentrated is something pH 3 vs something pH 6?5) Which will have more H+ concentration, a base with pH 11 or a base with pH 8? 6) A substance with pOH of 4 will have more H+ or OH-?
Ph And Poh Calculations Worksheet Answers Ph and Poh Calculations Chemistry Worksheet With Answers Download For Ph And Poh Calculations Worksheet Answers. If you want to add a word search puzzle, draw a textbox and write "wordsearch". Then enter the number of rows and columns and your wordsearch will seem. Adjust the scale if neccesary and click on the words.
PDF pH and pOH Worksheet - Mr. Smith's Website pH and pOH Worksheet Name: _____ Date: _____ Answer each question as completely as possible showing all work and units! 1) The concentration of either H+ ion or the OH- ion is given for three aqueous solutions at 298K. For each solution, calculate [H+] or [OH-]. State whether the solution is acidic, basic or neutral.







0 Response to "40 ph and poh worksheet"
Post a Comment