44 mixed gas law worksheet answers
Mixed Gas Laws Problems Worksheets - Learny Kids Mixed Gas Laws Problems Displaying top 8 worksheets found for - Mixed Gas Laws Problems. Some of the worksheets for this concept are Mixed gas laws work, Mixed gas laws work, Gas laws work, 3 gas laws and key, Mixed gas laws practice work name p, Extra practice mixed gas law problems answers, , Chemistry boyles and charless laws practice problems. DOC Mixed Gas Law Worksheet - Notre dame Chemistry solve the problem, and write your final answer on the line. Make sure you don't forget your units!!! 13.1. 1. The gas in a sealed can is at a pressure of 3.00 atm at 25(C. ... Worksheet - Mixed Gas Law Worksheet Author: faughtt Last modified by: Catherine Pala Created Date: 5/7/2019 7:42:00 PM Company: TOWNSHIP HIGH SCHOOL Other titles:
Early Ideas about Matter | Chemistry | Visionlearning Dalton made it a regular habit to track and record the weather in his hometown of Manchester, England. Through his observations of morning fog and other weather patterns, Dalton realized that water could exist as a gas that mixed with air and occupied the same space as air. Solids could not occupy the same space as each other; for example, ice could not mix with air.

Mixed gas law worksheet answers
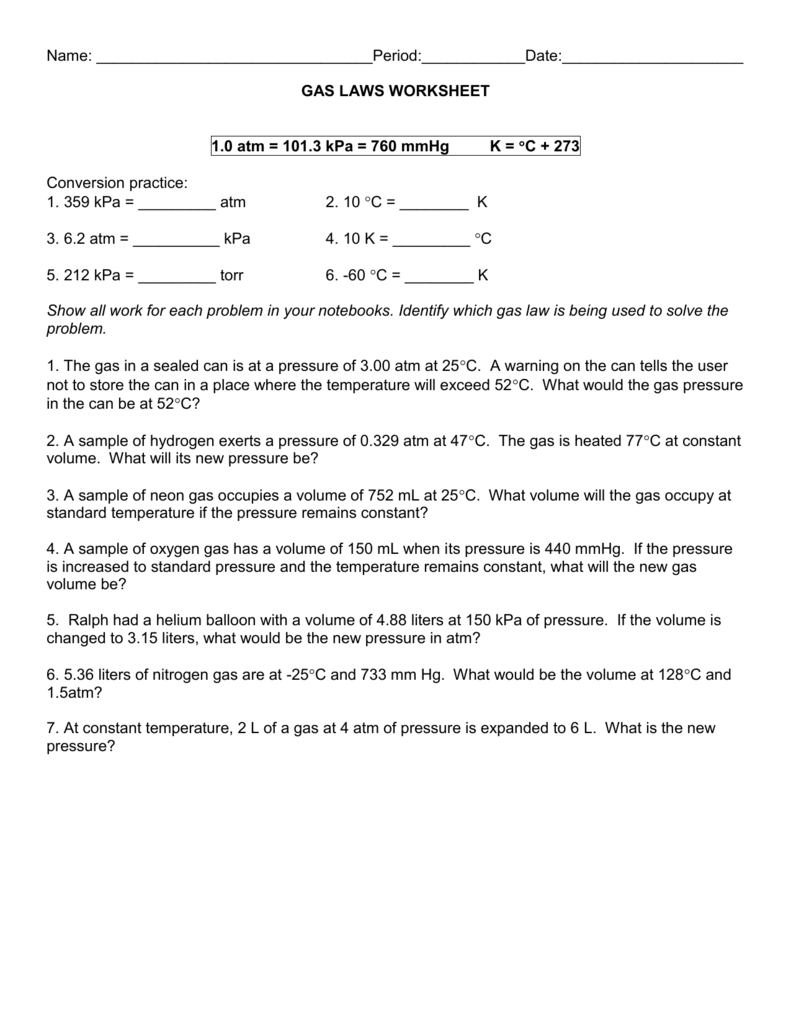
DOC MIXED GAS LAWS WORKSHEET - Liberty Union High School District write the formula of the gas law you plan to use to . solve each question. Show which values you have, which values are missing and/or which values need to be calculated. Be careful to use standard units of volume (liters), temperature (Kelvins) & pressure (Atm or mm of Hg). Note: 1 atm = 760 mm Hg. A gas occupies 3.5L at 2.5 mm Hg pressure. Ideal Gas Law Worksheet PV = nRT - Quia Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems: K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 L*kPa / (K*mole) 1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters ... PDF Mixed Gas Law Wk Key - Marlington Local Schools Worksheet - Mixed Gas Law Worksheet Name: SHOW ALL WORK FOR ALL PROBLEMS And OOC = 273 K 1. 1.0 atm = 101.3 kPa = 760 mmHg atm 1 OOC = Change the following units: 359 kPa -113 oc ... circle your final answer - and make sure you don't forget your units!!! 1. The gas in a sealed can is at a pressure of 3.0d atm at 250d. A warning on the can tells the
Mixed gas law worksheet answers. PDF Student Worksheet for Chemical Gas Laws - Science Learning Space Student Worksheet for Chemical Gas Laws Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Gas Laws Moles and Rates Boyle's Law: P 1 V 1 = P 2 V 2 Molar Mass: Σ 𝐴 K I𝑖𝑐 𝑖 ℎ P O 𝑖 𝑐 Q What is a Bunsen Burner? | Bunsen Burner Parts, Diagram 29/07/2021 · The Parts of a Bunsen Burner. The entire burner is typically made of metal and it burner sits on the bench via a sturdy base. The main gas inlet at the base of the burner is attached to gas nozzle ... DOC Socorro Independent School District : Answer each question below. Then . write the name of the gas law used t. o solve each question in the left margin next to each question. A gas occupies 3.5L at 2.5 mm Hg pressure. What is the volume at 10 mm Hg at the same temperature? A constant volume of oxygen is heated from 100(C to 185(C. The initial pressure is 4.1 atm. PDF Mixed Gas Laws Answers Pdf Free Download Mixed Gas Laws Answers Book Free Download PDF at Our eBook Library. This Book have some digitalformats such us : kindle, epub, ebook, paperbook, and another ... Mixed Gas Laws Worksheet - Honors ChemistryMIXED GAS LAWS WORKSHEET 1) How Many Moles Of Gas Occupy 98 L At A Pressure Of 2.8 Atmospheres And A
MIXED GAS LAWS WORKSHEET.docx - Name_ Date_ Period_ Mixed Gas Laws ... 2) If 5.0 moles of O2 and 3.0 moles of N2are placed in a 30.0 L tank at a temperature of 25oC, what will the pressure of the resulting mixture of gases be? 3) A balloon is filled with 35.0 L of helium in the morning when the temperature is 20.0oC. By noon the temperature has risen to 45.0oC. What is the new volume of the balloon? PDF Gas Law Worksheet - Mr. Midtgard's Chemistry Class Worksheet - Mixed Gas Law Worksheet Name: _____ SHOW ALL WORK FOR ALL PROBLEMS I. 1.0 atm = 101.3 kPa ... circle your final answer - and make sure you don't forget your units!!! 1. The gas in a sealed can is at a pressure of 3.00 atm at 25 C. A warning on the can tells the PDF Gas Laws Worksheet Answer Key - New Providence School District 1. Calculate the decrease in temperature when 6.00 L at 20.0 °C is compressed to 4.00 L. 2. A container containing 5.00 L of a gas is collected at 100 K and then allowed to expand to 20.0 L. What must the new temperature be in order to maintain the same pressure (as required by Charles' Law)? 3. A gas occupies 900.0 mL at a temperature of 27.0 °C. 1.2 Phases and Classification of Matter – Chemistry Both liquid and solid samples have volumes that are very nearly independent of pressure. A gas takes both the shape and volume of its container. Figure 1. The three most common states or phases of matter are solid, liquid, and gas. A fourth state of matter, plasma, occurs naturally in the interiors of stars. A plasma is a gaseous state of matter that contains appreciable numbers of ...
DOCX MIXED GAS LAWS WORKSHEET (modified by Mr. Jasmann) Ideal gas law answers 7)If I have 21 moles of gas held at a pressure of 78 atm and a temperature of 900 K, what is the volume of the gas? 19.8 L 8)If I have 1.9 moles of gas held at a pressure of 5 atm and in a container with a volume of 50 liters, what is the temperature of the gas? 1602 K DOC Mixed Gas Law Worksheet - St. Francis Preparatory School 1. The gas in a sealed can is at a pressure of 3.00 atm at 25(C. A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(C. What would the gas pressure in the can be at 52(C? 2. A sample of hydrogen exerts a pressure of 0.329 atm at 47(C. The gas is heated 77(C at constant volume. PDF Even More Gas Law Worksheet - St. Francis Preparatory School Worksheet - Mixed Gas Law Worksheet Name: _____ SHOW ALL WORK FOR ALL PROBLEMS I. 1.0 atm = 101.3 kPa ... circle your final answer - and make sure you don't forget your units!!! 1. The gas in a sealed can is at a pressure of 3.00 atm at 25 C. A warning on the can tells the The Law of Conservation of Mass: Definition, Equation & Examples Sep 23, 2021 · Two gas streams, having the flows rates and properties indicated in Table 4.7, are mixed in a pipeline. Assume perfect mixing, i.e. no change of volume upon mixing, and determine the composition of th
PDF Mixed Gas Laws Worksheet - Honors Chemistry MIXED GAS LAWS WORKSHEET - SOLUTIONS 1) How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K? n = PV = (2.8 atm)(98 L) RT (0.0821 L.atm/mol. K)(292 K) = 11 moles of gas 2) If 5.0 moles of O 2 0and 3.0 moles of N 2
Mixed Gas Laws Worksheet - Everett Community College Mixed Gas Laws Worksheet - Solutions 1) How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K? n = PV = (2.8 atm)(98 L) = 11 moles of gas RT (0.0821 L.atm/mol.K)(292 K) 2) If 5.0 moles of O 2 and 3.0 moles of N 2 are placed in a 30.0 L tank at a temperature of 25 0
Cathode Ray Experiment by JJ.Thomson (CRT) - Explanation ... Cathode Ray Tube - The Cathode Ray Experiment by J.J.Thomson helped to discover electrons. Cathode ray tube is the heart of the oscilloscope and it generates the electron bean, accelerates the beam and deflects the beam. Visit BYJUS to learn more about it.
DOC Gas Laws Worksheet #2: Boyle, Charles, and Combined Gas Laws Combined Gas Law Problems: 1 atm = 760.0 mm Hg = 101.3 kPa k = 273 +oC A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °C and the pressure is 740.0 mm of mercury. What will its volume be at 20.0 °C and 780 .0 mm of mercury pressure?
Cathode Ray Experiment by JJ.Thomson (CRT) - Explanation Cathode Ray Tube - The Cathode Ray Experiment by J.J.Thomson helped to discover electrons. Cathode ray tube is the heart of the oscilloscope and it generates the electron bean, accelerates the beam and deflects the beam. Visit BYJUS to learn more about it.
PDF Combined Gas Law Worksheet - Westgate Mennonite Combined Gas Law Worksheet - Solutions 1) If I initially have 4.0 L of a gas at a pressure of 1.1 atm, what will the volume be if I increase the pressure to 3.4 atm? (1.1 atm)(4.0 L) = (3.4 atm)( x L) x = 1.29 L 2) A toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 L.
The Ideal and Combined Gas Laws PV = nRT or P1V1 = P2V2 T 1 T2 The Ideal and Combined Gas Laws PV = nRT or P 1V 1 = P 2V 2 T 1 T 2 Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be PV = nRT 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Light and Color Review - Answers #2 - Physics Classroom The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.
Chemistry Study Guide for Gases - ThoughtCo Jul 03, 2019 · The values predicted by the ideal gas law are typically within 5% of measured real world values. The ideal gas law fails when the pressure of the gas is very high or the temperature is very low. The van der Waals equation contains two modifications to the ideal gas law and is used to more closely predict the behavior of real gases.
PDF Gas Law's Worksheet - Willamette Leadership Academy CHEMISTRY GAS LAW'S WORKSHEET 20. Determine the molar mass of a gas that has a density of 2.18 g/L at 66°C and 720 mm Hg. (Hint: the number of moles of a substance is its mass/molecular mass and density is mass/volume.) 19. What is the pressure in atm exerted by 2.48 moles of a gas in a 250.0 mL container at ...
The Law of Conservation of Mass: Definition, Equation & Examples 23/09/2021 · Two gas streams, having the flows rates and properties indicated in Table 4.7, are mixed in a pipeline. Assume perfect mixing, i.e. no change of volume upon mixing, and determine the composition of th






0 Response to "44 mixed gas law worksheet answers"
Post a Comment