44 quantum numbers practice worksheet
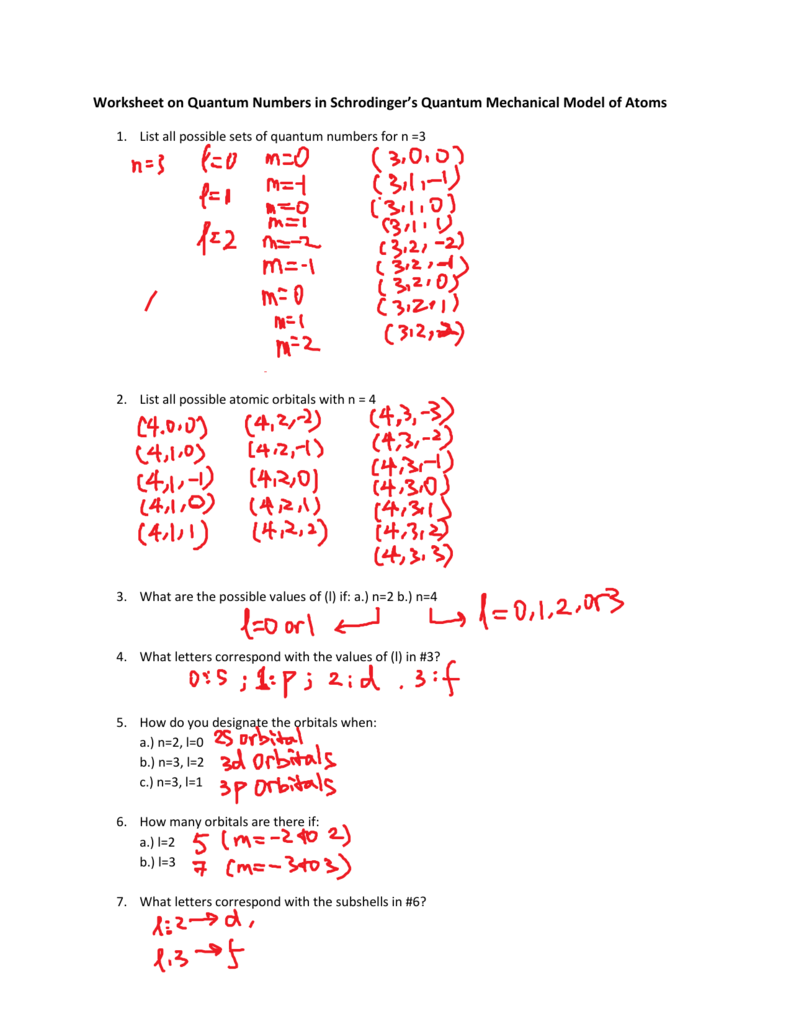
Quantum Numbers Notes and Practice Worksheet Quantum numbers can be difficult to teach. Use this graphic organizer to help structure your students' notes, and then check their understanding with the accompanying practice worksheet. This resource includes: • Quantum Numbers and What They Mean Student Handout (1 page) • Quantum Numbers and Wha... PDF QUANTUM NUMBERS WORKSHEET answers ms - Spin Quantum number: represents the electron and its spin. Two possibilities +1/2, -1/2 2. State the number of possible electrons described by the following quantum numbers a. n = 3, l = 0 2 b. n = 3, l = 1 6 c. n = 3, l = 2, ml = -1 2 d. n = 5, l = 0, ml -2, ms -1/2 Not possible
PDF Quantum numbers worksheet with answers - modaxch.com Quantum numbers worksheet with answers pdf. The main quantum number is represented by â â ̈̈"n has wavering values from â â ̈̈¢ â ̈̈̈¢ indince "â â ̈̈" The size and level of energy in which the electron is housed. The larger the N value, the more the electron is from the nucleus. The degeneration is when the orbitals have the same value as n.

Quantum numbers practice worksheet
Quantum Numbers Worksheets & Teaching Resources | TpT Quantum Numbers Worksheet - On this practice worksheet, students determine the noble gas configurations, draw orbital diagrams, find the quantum numbers for the last electron and complete lewis structures. This worksheet is organized and formatted to help students understand the process of finding Subjects: Chemistry, Physical Science, Science Quantum Numbers Worksheet copy.pdf - Name Quantum Number Practice ... View Quantum Numbers Worksheet copy.pdf from CHEM 151 at Chandler-Gilbert Community College. Name: _ Quantum Number Practice Worksheet Date: _ 1. Summarize: The principal quantum number, n, can have DOCX Quantum Numbers Practice Worksheet Given the element and quantum number: Noble-Gas Electron Configuration Element Identification Quantum Number [Rn] 7s2 5f4 U 5, 3, +1, +½ [Ar] 4s2 3d10 4p2 Ge 4, 1, 0, +½ [Ar] 4s2 3d 3 V 3, 2, 0, +½ [He] 2s1 Li 2, 0, 0, +½ [Kr] 5s2 4d3 Nb 4, 2, 0,+½
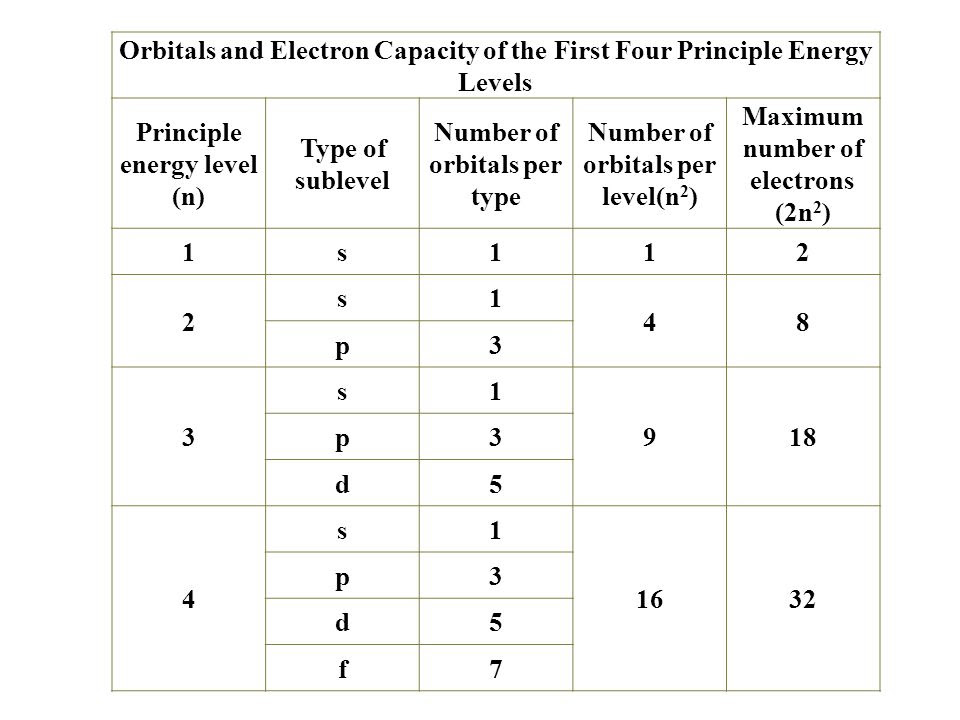
Quantum numbers practice worksheet. Quiz - Quantum Numbers The quantum mechanical model describes electrons as: particles waves particles with wave-like properties small, hard spheres 6. Which of the following sets is not an acceptable set of quantum numbers? n=2, l=1, ml=-1 n=7,l=3,ml=3 n=2,l=1,ml=1 n=3,l=1,ml=-3 7. Which atom has the largest atomic radius? Magnesium Barium Fluorine Mercury 8. PDF Quantum Number Practice Worksheet Answers Which of the following sets of quantum numbers are allowed for an electron in an orbital of a hydrogen atom: (a) n = 1, 1= 1, ml = 0 (b) n = 3, I = 0, ml = 0 (c) n = 4, 1, ml = -1 (d) n = 2, I = 1, ml = 2 Write the designation for the sublevel to w ich the orbital belongs. 11. PDF Quantum numbers worksheet pdf with answers Here obtain a quantum number of worksheet on the quantum number to excel during the examination. You might also want to look at our post on Quantum Numbers Chart. QUANTIC NUMBERS PRACTICE Problems Sheet question 1. What is the total number of orbitals associated with the main quantum number = 3? QUANTUM NUMBERS WORKSHEET - Justonly b. The subshell with the quantum numbers n=4, l=2 is ______. c. The ml values for a d orbital are .5 pages
Worksheet – Quantum Numbers Quantum Numbers Notes and Practice. This is our final way to describe the location of an electron. It consists of four numbers that act as coordinates to.2 pages 50 Quantum Numbers Practice Worksheet - Chessmuseum You can download and please share this 50 Quantum Numbers Practice Worksheet ideas to your friends and family via your social media account. Back to 50 Quantum Numbers Practice Worksheet Atom Structure Worksheet Middle School from quantum numbers practice worksheet , image source: Gallery of 50 Quantum Numbers Practice Worksheet Quiz & Worksheet - Principal Quantum Number | Study.com Worksheet 1. How many nodes are there for n = 4? 1 2 3 4 2. Which of the following statements about quantum numbers is FALSE? A smaller principal quantum number signifies a smaller electron cloud.... PDF Atomic Structure-Quantum Numbers -Lesson 2-Practice Questions 5. Indicate the quantum numbers for each of the orbitals of the sublevels indicated in the following chart: n l m a) 3 p sublevel b) 5 d sublevel 6. If one electron has the quantum numbers n = 4, l = 3, m = +2, s = - 1 2 and another electron has the quantum numbers n = 4, l = 3, m = -2, s = + 1 2, what are the
Quantum Number Practice Questions - JEE Chemistry MCQs The total number of electrons that can be accommodated in all the orbitals having principal quantum number 2 and azimuthal quantum number 1 is (a) 2 (b) 4 (c) 6 (d) 8 Ans. (c) Ques. When the azimuthal quantum number has a value of l = 1, the shape of the orbital is (a) Unsymmetrical (b) Spherically symmetrical (c) Dumb-bell (d) Complicated Ans ... PDF Quantum Numbers - Hudson City Schools / Homepage QUANTUM NUMBERS WORKSHEET Name 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number 3. Give the n and I values for the following orbitals a. Is d. 4d e. 5f 4. Circle all of the following orbital destinations that are theoretically pos .ble. b. Ip c. 5d d. 2d e 4f ... PDF QUANTUM NUMBERS WORKSHEET - lee.k12.nc.us QUANTUM NUMBERS WORKSHEET Name ________________________________ State the four quantum numbers and the possible values they may have. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0 Give the n and L values for the following orbitals a. 1s b. 3s c. 2p Quiz & Worksheet - Electron Configurations & the Four Quantum Numbers ... Worksheet 1. Which of the following is NOT true about the principal quantum number? It tells us the energy level of an electron. It tells us the size of an electron's orbital. It tells us the total...
PDF Orbitals and Quantum Numbers Practice Questions = 4 l = 1 3 (the 4p orbitals) = 2 l = 2 none (l must be < n) = 3 l = 2 5 (the 3d orbitals) = 5 l = 1 ml = -1 1 (3 q.n. define a unique orbital) 4. Which orbitals cannot exist? 2p 3p 4d 3f 6s 2d 3f and 2d 5. Write a set of quantum numbers for a 4f orbital. = 4 = 3ml = 3, 2, 1, 0, -1, -2, -3
PDF Worksheet - Quantum Numbers Give the four quantum numbers which describe the location of each of the following: 7. The 4th electron in C n=2, =0, m = -1 , ms = -1/2 8. The 5th electron in C n=2, =1, m = -1 , ms = +1/2 9. The 11th electron in Ar n=3, =0, m = 0 , ms = +1/2
DOC Worksheet - Quantum Numbers Quantum Numbers Practice Problems. Fill in the orbital notation (arrows) below, then write the four quantum numbers which describe the location of the highest energy (last) electron of the following elements: ... Worksheet - Quantum Numbers Author: Student Last modified by: 0 0 Created Date: 1/4/2016 1:35:00 PM Company:
PDF Quantum Number Practice Worksheet - Weebly What is the maximum number of electrons in an atom that can have the following quantum numbers: (a) n = 3 (b) n = 4, l = 2 (c) n = 4, l = 3, m l = 2 (d) n = 2, l = 1, m l = 0, m s = - ½ 13. The quantum numbers listed below are for four different electrons in the same atom. Arrange them in order of increasing energy.
Quantum Numbers Worksheets - K12 Workbook Displaying all worksheets related to - Quantum Numbers. Worksheets are Work quantum numbers, Quantum numbers work answers, Quantum numbers work, Name date quantum number practice work, Quantum numbers and electron configurations, Work quantum mechanics, Work 10, Quantum numbers and atomic orbitals.
PDF Name: Date: Quantum Number Practice Worksheet The quantum numbers listed below are for four different electrons in the same atom. Arrange them in order of increasing energy. Indicate whether any two have the same energy. (a) n = 4, l = 0, ml= 0, ms= ½ (b) n = 3, l = 2, ml= 1, ms= ½ (c) n = 3, l = 2, ml= -2, ms= -½ (d) n = 3, l = 1, ml= 1, ms= -½
PDF WORKSHEET Quantum Mechanics - Azle ISD / Homepage e. No two electrons in one atom can have the same four quantum numbers AP2. Which of the following represents the ground state electron configuration for the Mn3+ ion? 2 2a. 1s 2s 2p63s23p63d4 2 2b. 1s 2s 2p63s23p63d54s2 c. 1s 2p22s2 63s23p63d24s2 d. 1s 22s22p63s 23p63d84s 22e. 1s 2s 2p63s23p63d34s1 AP3. 3 1s 22s22p63s 3p
PDF Quantum Number Practice Worksheet - Tina's Science Class Quantum Number Practice Worksheet. 1. Summarize: The principal quantum number, n, can have the values of: 2 3 4 5, etc. 123. The angular momentum quantum ...2 pages




0 Response to "44 quantum numbers practice worksheet"
Post a Comment