45 5.1 models of the atom worksheet answers
PDF 5.1 Molecular Models for Methane Burning Worksheet Activity 5.1: Molecular Models for Methane Burning Worksheet A. Introduction Methane is sometimes called natural gas. It is found under the ground and is extracted to use as a fuel. Methane is a good fuel because it has chemical energy stored in its high-energy C-H bonds. When methane burns, it reacts with oxygen (O PDF 5.1 Models of the Atom 5 Section 5.1 Models of the Atom 127 5.1 Models of the Atom Aeronautical engineers use wind tunnels and scale models to simulate and test the forces from the moving air on each proposed design. The scale model shown is a physical model. However, not all models are physical. In fact, several theoretical models of the atom
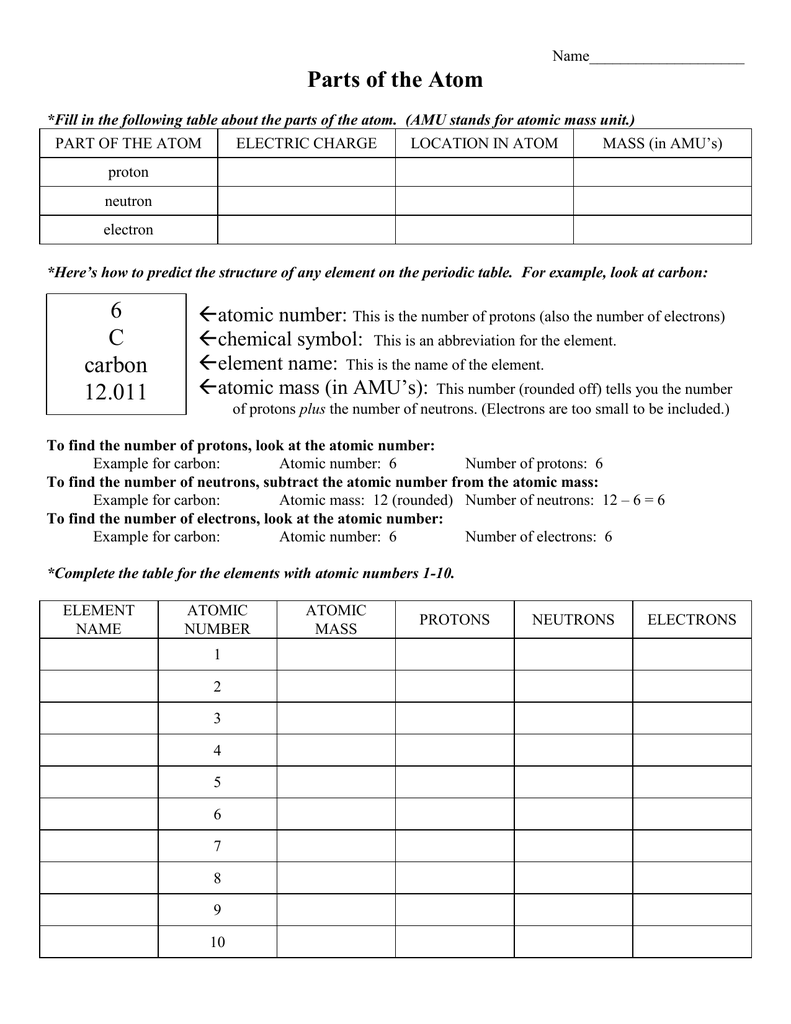
PDF Atomic Structure - Rim of the World Unified School District How many electrons are in the nucleus of an atom with an atomic number of 20? How many neutrons are in the nucleus of an atom with an atomic number of 25? (use Periodic Table for mass) What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an

5.1 models of the atom worksheet answers
5.1 Models of the Atom Section Review Flashcards | Quizlet Always True (AT) The electron probability clouds for atomic orbitals are spherical in shape Sometimes True (ST) The number of sublevels in an energy level is equal to the square of the principal quantum number of that energy level Never True (NT) The maximum number of electrons that can occupy the fourth principal energy level of an atom is 32 PDF Models of the Atom Homework - Answers - iTeachly.com What major step does the plum pudding model represent in terms of atomic research? It suggested that atoms were in fact divisible and provided the first evidence of subatomic particles, namely the electron. PDF Chapter 5.1 Revising the Atomic Model - Mr.Nguyen's Pre AP Chemistry Chapter 5.1 Revising the Atomic Model Page 140 5.1 Lesson Check #1-7 1. The Bohr model propose that an electron is found only in specific circular paths or orbits around the nucleus. Electrons in Bohr's model have specific energies. These specific energies of an electron are called energy levels. 2.
5.1 models of the atom worksheet answers. Worksheet 5.1 - Models of the Atom.pdf - Models of the Atom... View Homework Help - Worksheet 5.1 - Models of the Atom.pdf from ECON 101 at Omni College. Models of the Atom WS 5.1 Name:_ Per:_ Part I - Complete the following table of atomic models Name of PDF Atomic Structure Worksheet - WILLAMETTE LEADERSHIP ACADEMY 11. How many protons are in the nucleus of an atom with an atomic number of 15? 12. How many electrons are in the nucleus of an atom with an atomic number of 20? 13. How many neutrons are in the nucleus of an atom with an atomic number of 25? (use Periodic Table for mass) 14. What is the mass number of an atom with 3 protons, 4 neutrons, and 3 ... Solved 5.1 Stereochemistry Worksheet Naming Chirality | Chegg.com (Circle ane) H/CH/CHCH,/Br Which atom from the groups in the model has the highest atomic number? (Circle; Question: 5.1 Stereochemistry Worksheet Naming Chirality Centers - Rands Hipso 14 is back CHE = 0 Hi CH,CHE CH.CH . . Which group in the model above has the highest priority? Chemistry (12th Edition) Chapter 5 - Electrons in Atoms - GradeSaver Chapter 5 - Electrons in Atoms - 5.1 Revising the Atomic Model - 5.1 Lesson Check - Page 132: 5 Answer Quantized energies means that electrons are moved between energy levels by gaining or losing a certain amount of energy. Work Step by Step
CK-12 Chemistry Worksheet Name _____ Class _____ Date _____ Answer each of the questions below to show your achievement of the lesson objectives. Lesson Objective: Define chemistry. 1.Chemistry is the study of a.living systems b.the stars and planets c.all matter d.reactions in a test tube 5.1 Review - Name Date Class MODELS OF THE ATOM Section... Worksheet 5.1 - Models of the Atom.pdf. Omni College. ECON 101. homework. homework. Arlington High School ... Kami Export - MAYA SMITH - 5-1 Additional Practice.pdf. Leonardtown High. ENGLISH NO COURSE . Osbourn Park High School ... 5.1 models of the atom Flashcards | Quizlet the modern description of the behavior of electrons in atoms. Summarize the development of atomic theory ( short answer ) 1. Dalton proposed that matter was made of indestructible particles called Atoms. 2. Thompson's proposed that the atomic model in which negatively charged electrons were embedded in a positively charged Mass. 3. PDF Example Exercise 5.1 Atomic Notation - austincc.edu State the number of protons and the number of neutrons in an atom of each of the following isotopes. (a) (b) mercury-202. State the number of protons and the number of neutrons in an atom of each of the following isotopes. (a) (b) uranium-238. Answers: (a) 50 p + and 70 n. 0; (b) 92 p + and 146 n. 0. Practice Exercise
Chapter 5 electrons of an atom in its ground state into various orbitals around the nuclei of atoms • aufbau principle: the rule that electrons occupy the orbitals of lowest energy first • Pauli exclusion principle: an atomic orbital may describe at most two electrons, each with opposite spin direction PDF 5.1 Models of the Atom - Weebly Models of the Atom > The Quantum Mechanical Model The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. 5.1 . 11/18/14 8 PDF Models of the Atom Homework - Answers - iTeachly.com 1. How are electrons arranged in an atom? a. In groups of five b. By shape c. In energy levels d. By color 2. Name the scientist who used an alpha-particle scattering experiment to investigate the structure of the nucleus in the atom. a. John Dalton b. James Chadwick c. Ernest Rutherford d. Lord Kelvin 3. Where does the majority of an atom's ... The Nuclear Atom | CIE IGCSE Physics Questions & Answers 2020 (Medium ... 5.1 The Nuclear Atom Medium Hard Download PDF Quick Answers 1 2 3 4 5 6 7 8 Question 1 Marks: 1 Choose your answer A B C D View Answer Next Question 1. General Physics 2. Thermal Physics 3. Properties of Waves, including Light & Sound 4. Electricity & Magnetism 5. Atomic Physics 5.1 The Nuclear Atom 5.2 Radioactivity
PDF Chapter 5.1 Revising the Atomic Model - Mr.Nguyen's Pre AP Chemistry Chapter 5.1 Revising the Atomic Model Page 140 5.1 Lesson Check #1-7 1. The Bohr model propose that an electron is found only in specific circular paths or orbits around the nucleus. Electrons in Bohr's model have specific energies. These specific energies of an electron are called energy levels. 2.
PDF Models of the Atom Homework - Answers - iTeachly.com What major step does the plum pudding model represent in terms of atomic research? It suggested that atoms were in fact divisible and provided the first evidence of subatomic particles, namely the electron.
5.1 Models of the Atom Section Review Flashcards | Quizlet Always True (AT) The electron probability clouds for atomic orbitals are spherical in shape Sometimes True (ST) The number of sublevels in an energy level is equal to the square of the principal quantum number of that energy level Never True (NT) The maximum number of electrons that can occupy the fourth principal energy level of an atom is 32


![Atomic Structure Worksheet[1].doc - Atomic Structure Worksheet Name the ...](https://www.coursehero.com/thumb/30/16/3016d9f7923ea0749f8182fd73ceafc89bae0487_180.jpg)



0 Response to "45 5.1 models of the atom worksheet answers"
Post a Comment