40 percent composition and empirical formula worksheet
Object Identifier System This is the web site of the International DOI Foundation (IDF), a not-for-profit membership organization that is the governance and management body for the federation of Registration Agencies providing Digital Object Identifier (DOI) services and registration, and is the registration authority for the ISO standard (ISO 26324) for the DOI system. › mathsMaths | Learn Concepts and Formula-Based Questions - VEDANTU Maths | Learning concepts from basic to advanced levels of different branches of Mathematics such as algebra, geometry, calculus, probability and trigonometry. Also, understanding definitions, facts and formulas with practice questions and solved examples.
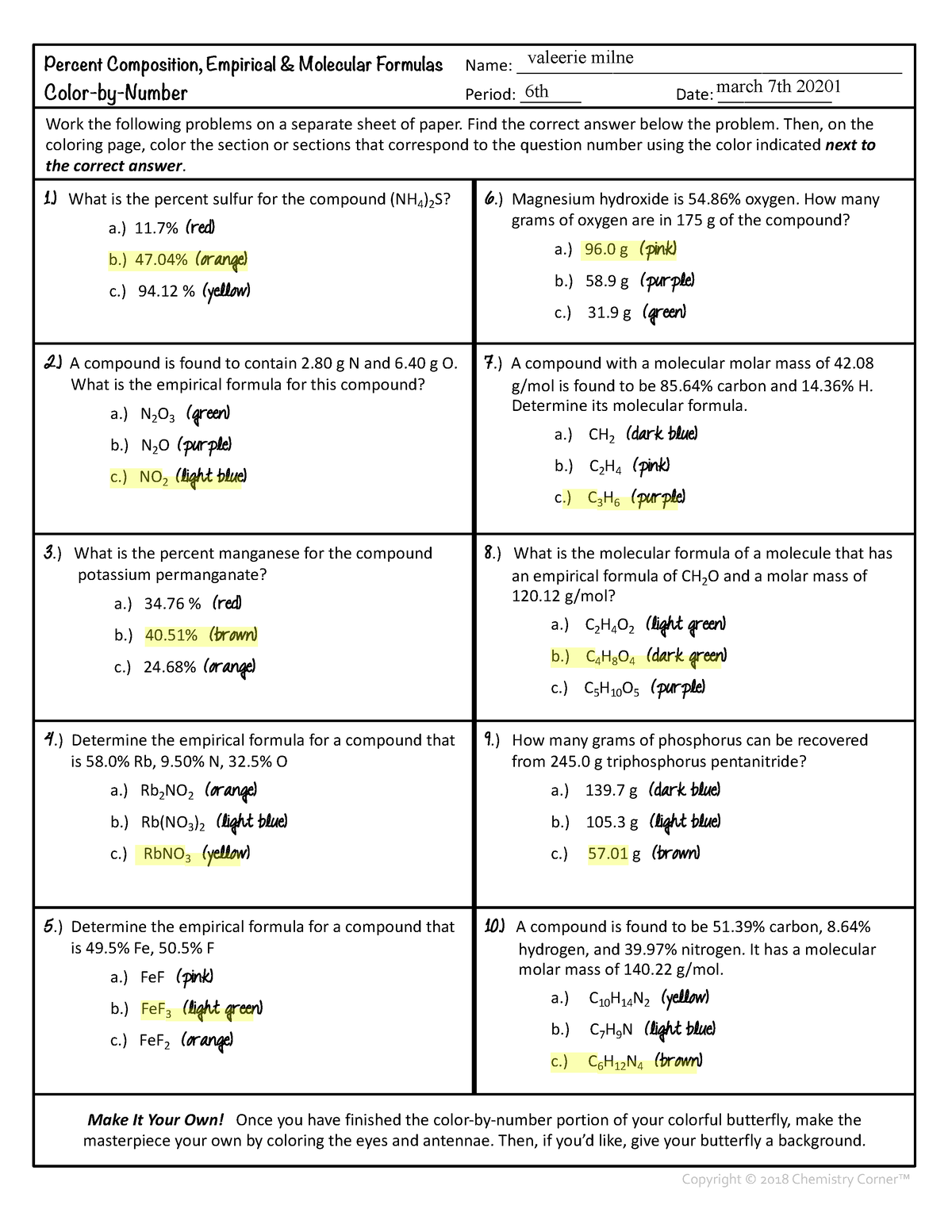
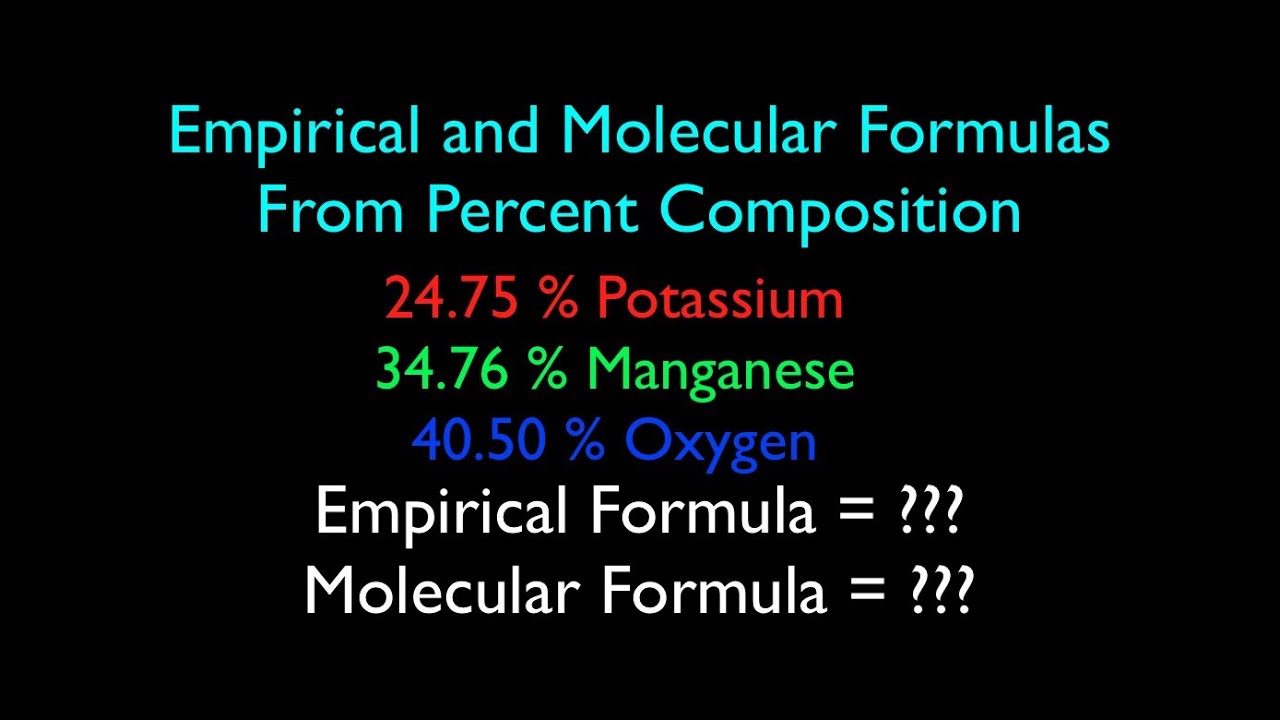
chemunlimited.com › 1111. Empirical Formula Worksheet II - chemunlimited.com EMPIRICAL AND MOLECULAR FORMULA WORKSHEET An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical formula. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula.

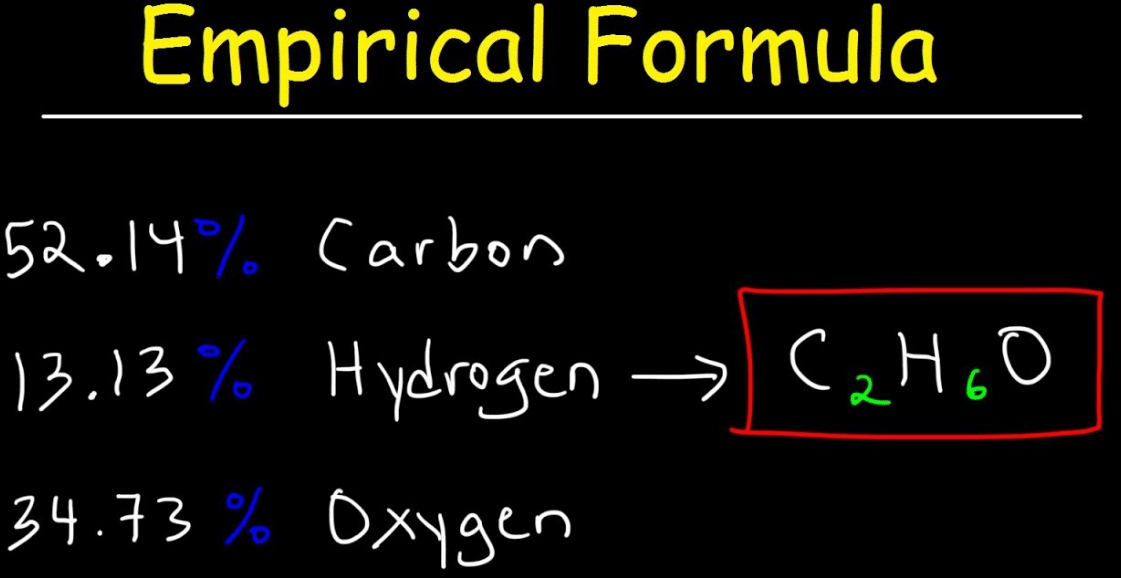
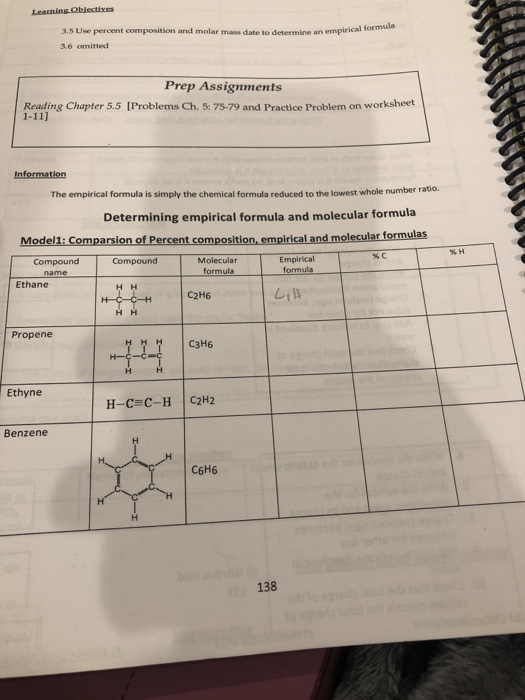
Percent composition and empirical formula worksheet
successessays.comSuccess Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. allinonehomeschool.com › step-3-2020-updatePre-Algebra – Easy Peasy All-in-One Homeschool That’s the percent. 1/2 = .5 Multiply by 100 and that’s 50 One half is 0.50, which is 50%. You can use a calculator. Find the percent for each fraction. See the correlation between the fraction, the picture, and the percent. They are all equal. Fill in 23 percent. How many would that be? That’s 23 out of 100. Look at this one more time. chem.libretexts.org › Courses › College_of_Marin7.11: The Activity Series - Chemistry LibreTexts May 20, 2018 · Chapter 6: Chemical Composition; 6.1: How Much Sodium? 6.2: Counting Nails by the Pound; 6.3: Counting Atoms by the Gram; 6.4: Counting Molecules by the Gram; 6.5: Chemical Formulas as Conversion Factors; 6.6: Mass Percent Composition of Compounds; 6.7: Mass Percent Composition from a Chemical Formula; 6.8: Calculating Empirical Formulas for ...
Percent composition and empirical formula worksheet. › mass-percent-compositionHow to Calculate Mass Percent Composition - ThoughtCo Nov 24, 2019 · Mass percent composition is also known percent by weight. It is abbreviated as w/w%. For a solution, mass percent equals the mass of an element in one mole of the compound divided by the molar mass of the compound, multiplied by 100%. chem.libretexts.org › Courses › College_of_Marin7.11: The Activity Series - Chemistry LibreTexts May 20, 2018 · Chapter 6: Chemical Composition; 6.1: How Much Sodium? 6.2: Counting Nails by the Pound; 6.3: Counting Atoms by the Gram; 6.4: Counting Molecules by the Gram; 6.5: Chemical Formulas as Conversion Factors; 6.6: Mass Percent Composition of Compounds; 6.7: Mass Percent Composition from a Chemical Formula; 6.8: Calculating Empirical Formulas for ... allinonehomeschool.com › step-3-2020-updatePre-Algebra – Easy Peasy All-in-One Homeschool That’s the percent. 1/2 = .5 Multiply by 100 and that’s 50 One half is 0.50, which is 50%. You can use a calculator. Find the percent for each fraction. See the correlation between the fraction, the picture, and the percent. They are all equal. Fill in 23 percent. How many would that be? That’s 23 out of 100. Look at this one more time. successessays.comSuccess Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.











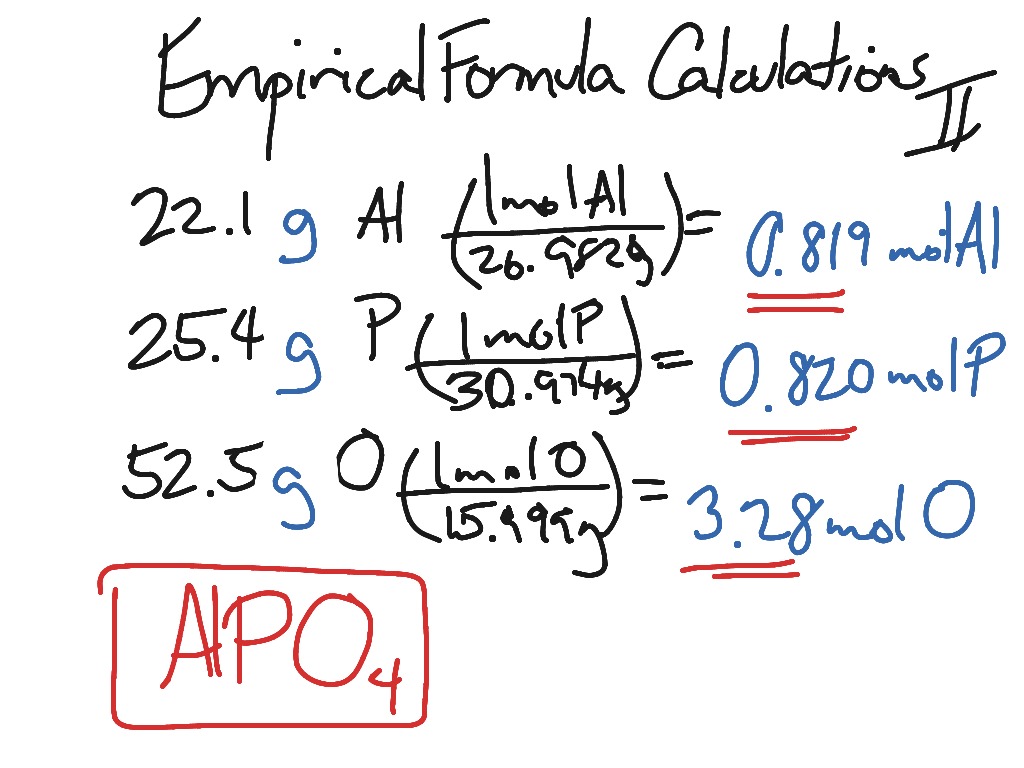

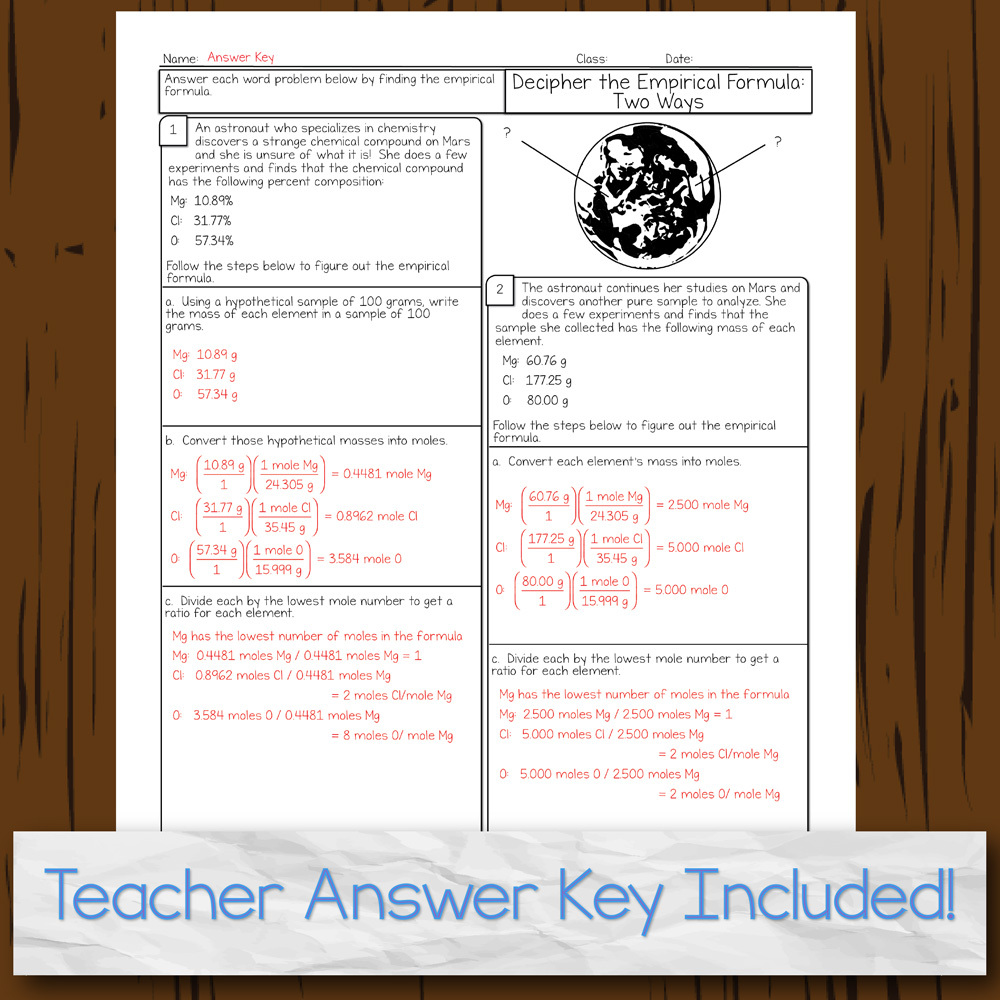
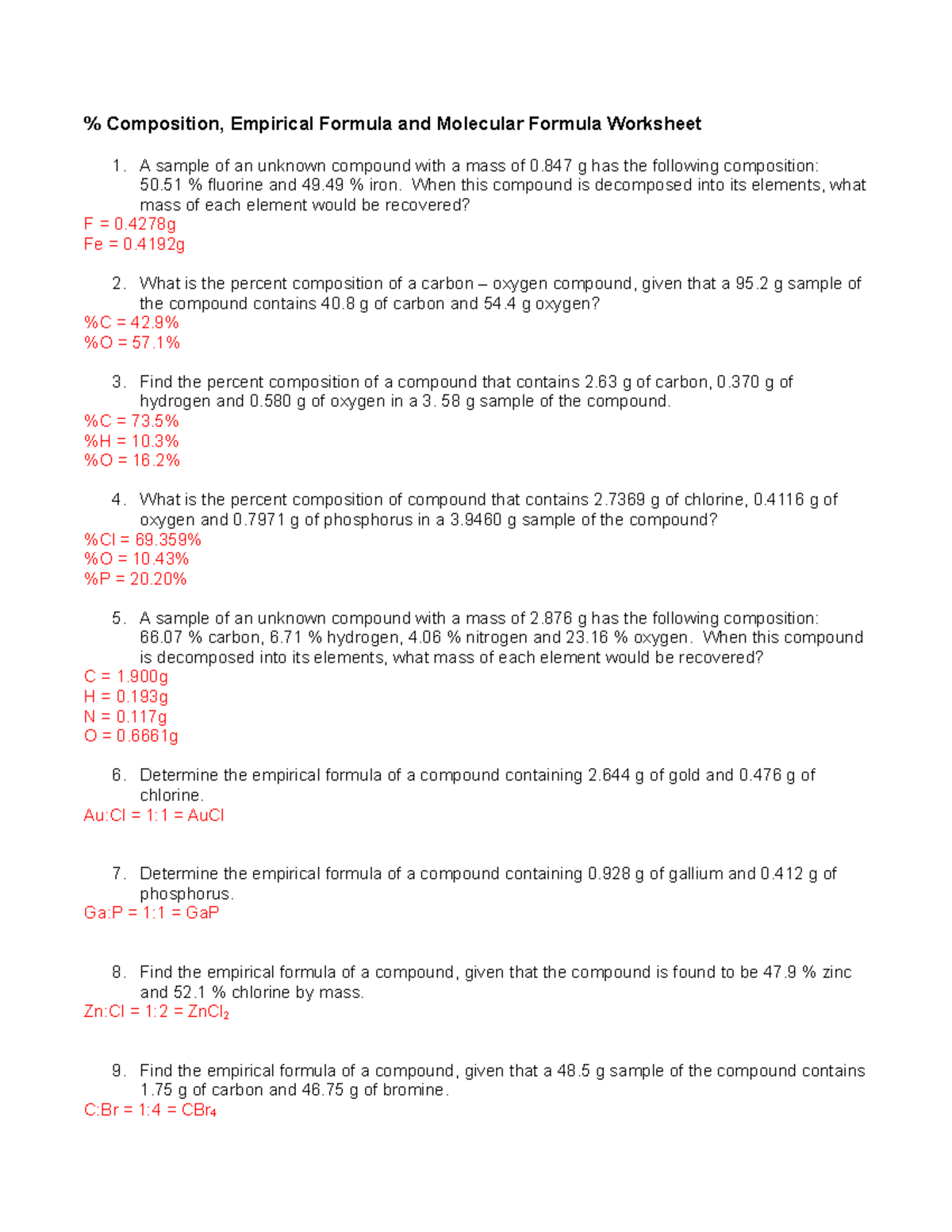




0 Response to "40 percent composition and empirical formula worksheet"
Post a Comment