41 calorimetry practice worksheet answers
Heat curve Practice Sheet.doc - Heating Curve/Calorimetry Worksheet ... 8) When a given quantity of water is heated at a constant rate, the phase change from liquid to gas takes longer than the phase change from solid to liquid because a. The heat of vaporization is greater than the heat of fusion b. The heat of fusion is greater than the heat of vaporization c. The average kinetic energy of the molecules is greater in steam than in water d. chemistrysky.com › Practice ProblemsChemistry and More - Practice Problems with Answers Dec 08, 2020 · From the Chem Team: Worksheet of mass mole conversions Answers to Worksheet of mass mole conversions . Reactions in Aqueous Solutions. Study Questions; Answers. More Study Questions; Answers. Practice Problems: Determining whether a precipitate forms; Answers . Atomic Structure and Periodicity. Study Questions; Answers. Electron configuration ...
PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q
Calorimetry practice worksheet answers
PDF Calorimetry Worksheet W 337 - Everett Community College 2) When 62.3 g of a compound was burned in a bomb calorimeter that contained 0.500 L of water the temperature rise of the water in the calorimeter was 48.00 C. If the heat of combustion of the compound is 1,160 kJ/mol, what is the molar mass of the compound? 0.500 L H 2 O = 500 mL H 2 O = 5.00 x 10 2 g H 2 O H = (5.00 x 102 g H 2 O)(4.184 J/g Heat capacity and calorimetry (practice) | Khan Academy Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for AP Chemistry students. PDF Calorimetry Problems Worksheet - bremertonschools.org 6. A 13.5 g sample of gold is heated, then placed in a calorimeter containing 60.0 g of water. Temperature of water increases from 19.00 oC to 20.00 oC. The specific heat of gold is 0.130 J/goC. What was the initial temperature of the gold metal sample? 7. A 28.4 g sample of aluminum is heated to 39.4 oC, then is placed in a calorimeter
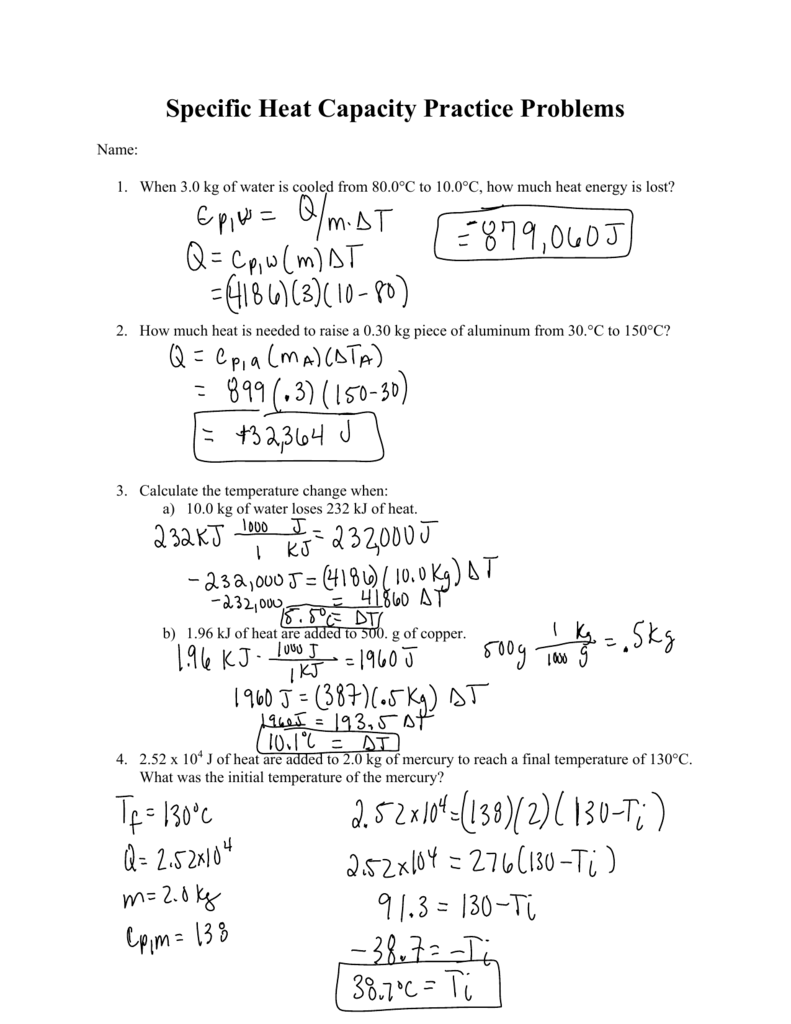
Calorimetry practice worksheet answers. PDF Calorimetry: Measuring the Energy in Foods - Carolina.com Calorimetry: Measuring the Energy in Foods A Carolina Essentials™ Investigation Data and Observations Student answers will vary in mass, and the final temperature of the water will vary with the type of food burned. Sample #1 Sample #2 Sample #3 Mass of Water 50 g 49 g 50 g Initial Mass of Food Sample and Paper Clip 4.2 g 3.7 g 3.2 g Calorimetry Practice Answer Key Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3. › lifestyleLifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing Quiz & Worksheet - Calorimetry | Study.com Choose an answer and hit 'next'. You will receive your score and answers at the end. question 1 of 3 Express 55.3 J of heat (or energy) in units of calories. 13.2 cal 231.4 cal .076 cal Not...
chemistry.uoregon.edu › profile › tgreenboThomas Greenbowe | Department of Chemistry and Biochemistry Oct 16, 2017 · 2015–present Senior Instructor II, University of Oregon. 2013–2015 Morrill Professor, Iowa State University. 1998-2013 Professor of Chemistry, Iowa State University. 2013-2014 Visiting Lecturer, University of Oregon. 2006 Visiting Professor, University of Arizona. 1990-1998 Associate Professor, Iowa State University, 1988-1990 Associate Professor of Chemistry and Director of Freshman ... PDF Calorimetry Answers - University of Illinois Urbana-Champaign 3. The heat of combustion of sucrose (C12H24012) in a bomb calorimeter is -3840 kJ/mol. / C12H22011 (S) + Balance the reaction. 02 (g) C02 (g) + 1-120 (g) When 20 grams of sucrose is burned, the temperature of the calorimeter rises by 9. PC. What is the heat capacity (C) of the calorimeter? (molar mass of sucrose = 360.3 g/mol) 5 57 qsys — Calorimeter Questions - Practice Questions with Answers & Explanations Important Calorimeter Questions with Answers 1) What is a calorimeter? A calorimeter is an apparatus used for calculating the heat developed during a chemical, mechanical or electrical reaction. It also helps to measure the heat capacity of various materials. 2) What is the principle behind a calorimeter? calorimetry worksheet 1 answers Calorimetry Worksheet Answers : Chapter 5 Thermochemistry / Back to. 11 Images about Calorimetry Worksheet Answers : Chapter 5 Thermochemistry / Back to : 26 Chemistry Worksheet Heat And Calorimetry Problems - Notutahituq, Specific Heat Worksheet #2 Answer Key - kidsworksheetfun and also Chemistry : Calorimetry Practice Worksheet.
DOC Calorimetry Problems 1 Chemistry: Calorimetry Problems 1 Solve the following problems. As always, include work and show the units to ensure full credit. 1. A 445 g sample of ice at -58oC is heated until its temperature reaches -29oC. Find the change in heat content of the system. 2. A 152 g sample of ice at -37oC is heated until it turns into liquid water at 0oC. PDF Bomb Calorimetry Practice Problems - crestwoodschools.org Created Date: 2/19/2016 7:33:05 AM Thermochemistry Worksheet + Answers - ChemistNate Step 1: Download this workbook which contains full solutions: . Thermochemistry-Worksheets-with-Full-Solutions-ChemistNate-July-2021.pdf. Download File. Step 2: Do the questions, and follow along with this video for when you get stuck: Study With Me: 90 Minutes of Thermo/Enthalpy/Heat Practice. Calorimetry Practice Problem Worksheets - K12 Workbook Chemistry Worksheet Heat And Calorimetry Problems Answers. 4. Calorimetry practice problems answers. 5. Thermochemistry Worksheet #1. 6. Calorimeter Practice With Answers. 7. Calorimetry practice problems with answers.
PDF Calorimetry and Molar Enthalpy Worksheet Answer Key - Onstudy Academy Calorimetry and Molar Enthalpy Worksheet Answer Key 1) 9.0 grams of charcoal (C) were completely consumed in a bomb calorimeter. If we assume that the 2.0 L of water absorbed all of the heat released by the charcoal, and if the temperature of the water increased from 20.25 ºC to 56.04 ºC, what is the molar enthalpy of carbon? Q water = m. c. T
☑ Calorimetry Worksheet Answers Calorimetry worksheet 1 if 0315 moles of hexane c 6 h 14 is combusted in a bomb calorimeter containing 565 liters of water calculate the m...
Calorimetry Problems 1 Worksheet Answer Key Calorimetry Problems 1 Worksheet Answer Key | added by request 832 kb/s 10187 Period - Chemmybear.com Measuring heat (formerly measured in calories) is called calorimetry. Now we measure heat energy ... 1. Water has a specific heat capacity of 4.184 J/g. °C.
PDF Calorimetry Worksheet - Laney College calorimeter, a 3.62 °C rise in temperature occurs. When a 0.305 g sample of citric acid, C 6 H 8 O 7, is burned, a 1.83 °C temperature rise is observed. If the heat of combustion of benzoic acid is 26.38 kJ/g, what is the molar heat of combustion of citric acid? First you need to calculate the heat capacity of the bomb calorimeter.
Calorimetry Class-10 Goyal Brothers ICSE Physics Solutions Ch-4 Answer: Practice Problems 7. Question 1. Calorimetry Class-10 Goyal Brothers What mass of a solid of specific heat capacity 0.75 Jg-10 C-1 will have heat capacity 93.75 Jg-1 °C-1 ? Answer: Question 2. A solid of mass 1.2 kg has sp. heat capacity of 1.4 Jg-1 °C-1. Calculate its heat capacity in SI units. Answer: Practice Problems 8. Question 1.
softmath.com › math-com-calculator › addingAlgebrator online - softmath Prentice Hall Algebra 2 with Trigonometry even answers ; adding and subtracting negative numbers worksheet ; quadratic formula calculator with complex roots ; math lesson plan instructions changing a decimal into a fraction ; sats yr6 questions for kids ; Aptitude questions +download ; trig equation solver ; math worksheet teat creator "free ...
physics.info › motion-graphs › practiceGraphs of Motion - Practice – The Physics Hypertextbook The third and fourth methods use the other two equations of motion. Since these rely on our choices for the final velocity, multiple valid answers are possible. Let's say we use the velocity calculated from the slope of a "tangent" with a value of −60 m/s and and the velocity-time relationship, a.k.a. the first equation of motion. Then…
› cms › libCalorimetry Practice Problems Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3.
openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. Methane and oxygen react to yield carbon dioxide and ...
PDF II Calorimetry Worksheet - GEOCITIES.ws (6) A 1.567 g sample of naphthalene, C 10H 8 (s), is completely combusted in a bomb calorimeter assembly and a temperature increase of 8.37 0C is observed. When a 1.227 g sample of thymol, C 10H 14O (s) (a preservative and a mold and mildew preventative), is burned in the same calorimeter assembly, the temperature increase is 6.12 0C. If the ...
Heat Capacity Calorimetry Worksheet answers - StuDocu For the same reasoning as question # 1 It takes less energy to raise 1 gram of a material's temperature with a low specific heat, where as 100 J of energy would have less of an effect on a material with a high heat capacity. A 5 g mass of a metal was heated to 100 oC and then plunged into 100 g of water at 24 oC.
Calorimetry Problems Worksheet Answer Key - qstion.co calorimetry worksheet 1 if 0315 moles of hexane c 6 h 14 is combusted in a bomb calorimeter containing 565 liters of water calculate the molar heat of combustion of hexane if the water temperature rises 554 c. calorimetry worksheet answer key posted on september 15, 2021 august 13, 2021 by admin allowed to our blog, with this occasion i am going …
Calorimetry Quiz : ChemQuiz.net This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅ΔT) with options for different units of heat and temperature. Select your preferences below and click 'Start' to give it a try!
Calorimetry Practice Worksheet Answers - 1 - - Gallery Briner Calorimetry Practice Worksheet Answers - 1 - It shows you how to calculate the quantity of . Temperature of water increases from 20.00 oc to 22.50 oc. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by.
PDF Calorimetry Practice Worksheet - mrsgingrashths.weebly.com Calorimetry Practice Worksheet 1) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.0 0 C, what is the molar heat of combustion o f compound A? The heat capacity of water is 4.184 J / g 0C.
PDF Name: Period: Seat# Worksheet #3 . Dougherty Valley HS Chemistry Thermochemistry - Calorimetry Practice Mathematical Questions ... incorrect answer. 11) In a coffee-cup calorimeter, 100.0 g of H 2 O and 100.0 mL of HCl are mixed. They both had an initial temperature of 24.6°C. After the reaction, the temperature of both substances is 31.3°C.
PDF Calorimetry Problems 1 6. If a 348 g sample of steam at 127oC is cooled to 103oC, find the change in heat content of the system. 7. In going from ice at -34oC to steam at 138oC, a sample of water absorbs 1.41 x 105 J. Find the mass of the sample. Answers: 4 1. 2.68 x 10 5J 2. 6.23 x 104 5J 3. -6.71 x 105 J 4. -1.11 x 10 J 5. 2.11 x 10 J 6.
PDF Calorimetry Problems Worksheet - bremertonschools.org 6. A 13.5 g sample of gold is heated, then placed in a calorimeter containing 60.0 g of water. Temperature of water increases from 19.00 oC to 20.00 oC. The specific heat of gold is 0.130 J/goC. What was the initial temperature of the gold metal sample? 7. A 28.4 g sample of aluminum is heated to 39.4 oC, then is placed in a calorimeter
Heat capacity and calorimetry (practice) | Khan Academy Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for AP Chemistry students.
PDF Calorimetry Worksheet W 337 - Everett Community College 2) When 62.3 g of a compound was burned in a bomb calorimeter that contained 0.500 L of water the temperature rise of the water in the calorimeter was 48.00 C. If the heat of combustion of the compound is 1,160 kJ/mol, what is the molar mass of the compound? 0.500 L H 2 O = 500 mL H 2 O = 5.00 x 10 2 g H 2 O H = (5.00 x 102 g H 2 O)(4.184 J/g

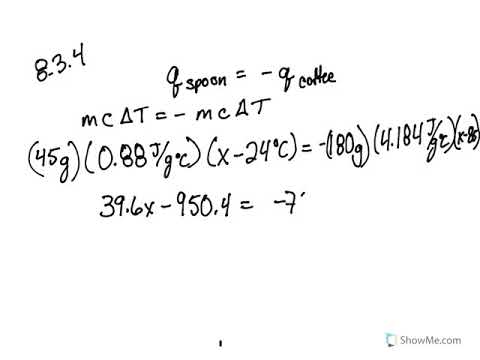


























0 Response to "41 calorimetry practice worksheet answers"
Post a Comment