45 specific heat worksheet answers
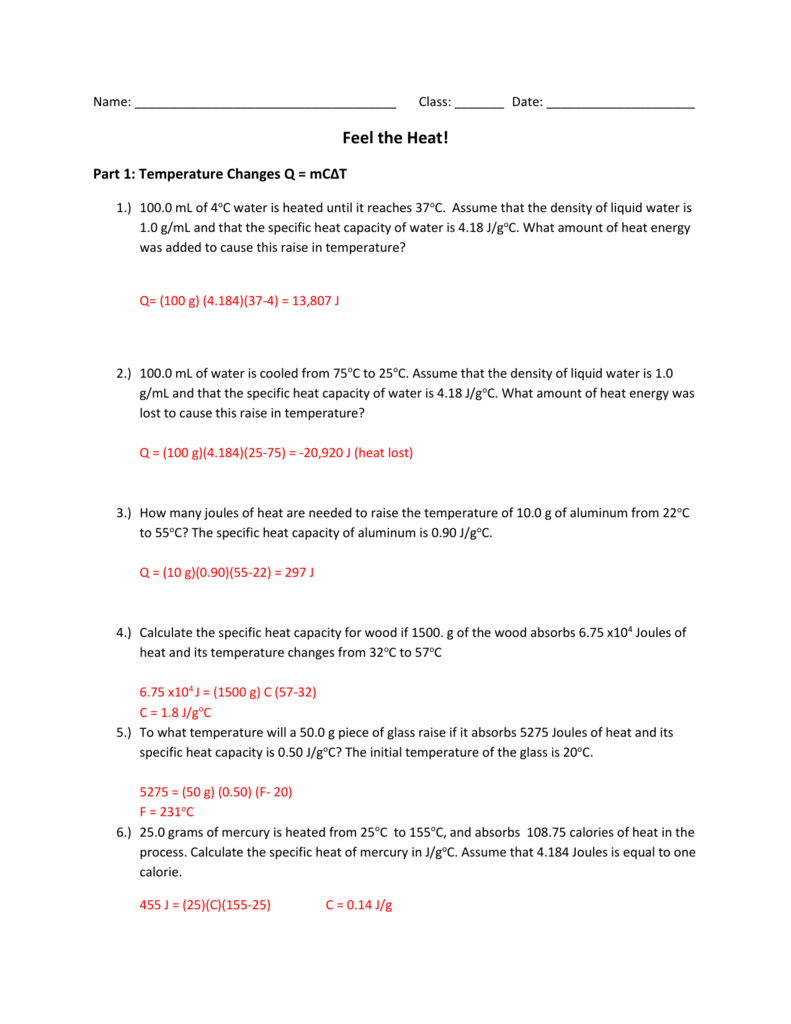
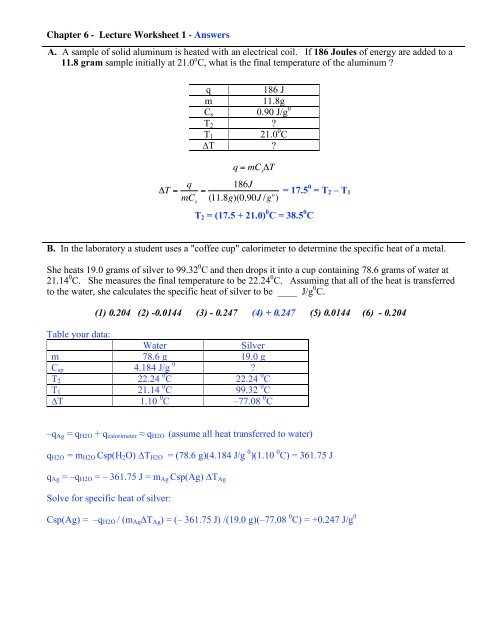
Specific Heat Worksheet q=(mass)(Csp)(AT) 1. What is the specific heat of a substance that absorbs 2.5 x 10³ joules of heat when a sample of 1.0 x 104 g of the substance increases in temperature from ...4 pages Specific Heat Worksheet Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ...2 pages
PDF Specific Heat Capacity Questions and Answers - chemistry! Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C ...5 pages

Specific heat worksheet answers
Specific Heat Chemistry this data, calculate the specific heat of aluminum. Check your answer with a specific heat table. 9. 100.0 mL of 4.0°C water is heated until its temperature ...4 pages Worksheet- Calculations involving Specific Heat Worksheet- Calculations involving Specific Heat. 1. For q= m ○c ○ Δ T : identify each variables by name & the units associated with it.2 pages Calculating Specific Heat Extra Practice Worksheet - TSFX If the specific heat of copper is 390 J/g 0C, what is the change of the copper's temperature? Page 4. Answers. Q = mc∆T, where Q = heat energy, ...5 pages
Specific heat worksheet answers. Specific Heat Worksheet Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ... Specific Heat And Heat Capacity Worksheet With Answers Pdf Specific Heat and Heat Capacity Worksheet 1. The temperature of 335 g of water changed from 24.5oC to 26.4oC. How much heat did this sample absorb? c for .1. A ... specific heat problems answer key.pdf - ISD 622 Specific Heat Worksheet. Rey. Cp = q/mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat ...1 page Calculating Specific Heat Extra Practice Worksheet - TSFX If the specific heat of copper is 390 J/g 0C, what is the change of the copper's temperature? Page 4. Answers. Q = mc∆T, where Q = heat energy, ...5 pages
Worksheet- Calculations involving Specific Heat Worksheet- Calculations involving Specific Heat. 1. For q= m ○c ○ Δ T : identify each variables by name & the units associated with it.2 pages Specific Heat Chemistry this data, calculate the specific heat of aluminum. Check your answer with a specific heat table. 9. 100.0 mL of 4.0°C water is heated until its temperature ...4 pages




























0 Response to "45 specific heat worksheet answers"
Post a Comment