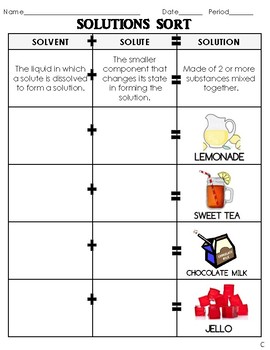
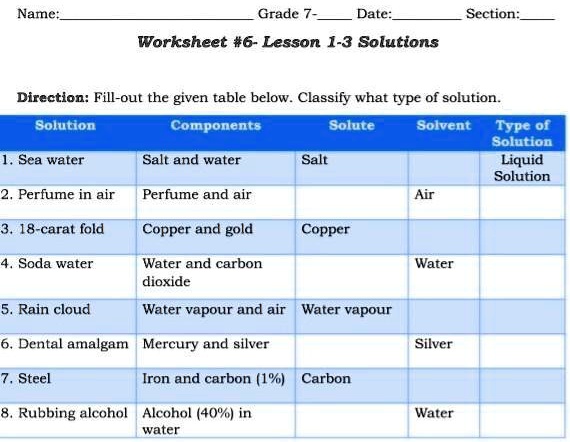
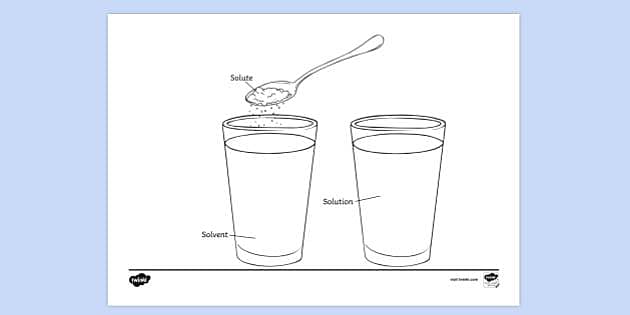
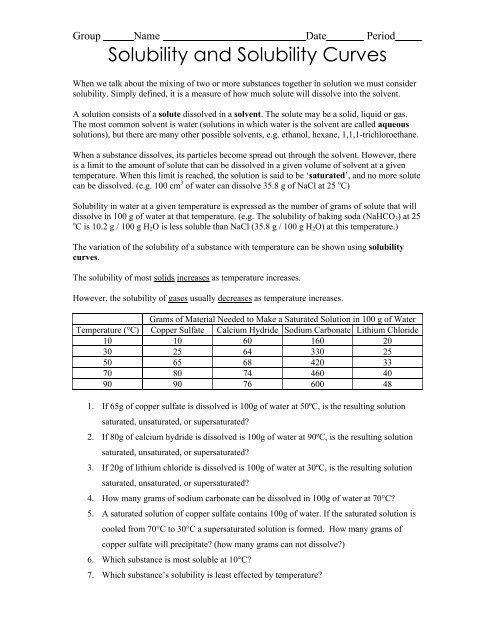
41 solute and solvent worksheet
Chemistry for Kids: Separating Mixtures - Ducksters Painting uses the separation process of evaporation. The wet paint is a mixture of color pigment and a solvent. When the solvent dries and evaporates, only the color pigment is left. The separation process of winnowing was used in ancient cultures to separate the grain from the chaff. Water - Wikipedia Water (H 2 O) is a polar inorganic compound.At room temperature it is a tasteless and odorless liquid, nearly colorless with a hint of blue.This simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances.
PHSchool.com Retirement–Prentice Hall–Savvas Learning Company PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support.

Solute and solvent worksheet
Molal Solution Concentration Calculator - PhysiologyWeb Jun 01, 2005 · m solvent is mass of solvent in kilograms (kg) in which the indicated mass of solute (m solute) in grams (g) must be dissolved to make the desired molal concentration (C m). MW is the molecular weight in g/mol. Molecular weight is also referred to as formula weight and, in fact, many scientists prefer to use the latter. (OLD VIDEO) Osmosis - YouTube This video has been updated here: Learn osmosis with real life examples! The terms hypertonic, hypotonic, and isotonic are explo... Numerical Problems on Percentage by Mass - The Fact Factor Jan 29, 2020 · A solution is prepared by dissolving a certain amount of solute in 500 g of water. The percentage by mass of a solute in a solution is 2.38. Calculate the mass of solute. Given: Mass of solvent = 500 g, percentage by mass = 2.38. To Find: Mass of solute =? Solution: Let the mass of solute = x g
Solute and solvent worksheet. Construction of the Cell Membrane - Wisc-Online OER Semi-permeable means "allowing certain substances to pass through it but not others, especially allowing the passage of a solvent but not of certain solutes." The cell membrane allows some things to pass through, and not others, which is why it needs transport proteins to let the other things through that the cell needs. Numerical Problems on Percentage by Mass - The Fact Factor Jan 29, 2020 · A solution is prepared by dissolving a certain amount of solute in 500 g of water. The percentage by mass of a solute in a solution is 2.38. Calculate the mass of solute. Given: Mass of solvent = 500 g, percentage by mass = 2.38. To Find: Mass of solute =? Solution: Let the mass of solute = x g (OLD VIDEO) Osmosis - YouTube This video has been updated here: Learn osmosis with real life examples! The terms hypertonic, hypotonic, and isotonic are explo... Molal Solution Concentration Calculator - PhysiologyWeb Jun 01, 2005 · m solvent is mass of solvent in kilograms (kg) in which the indicated mass of solute (m solute) in grams (g) must be dissolved to make the desired molal concentration (C m). MW is the molecular weight in g/mol. Molecular weight is also referred to as formula weight and, in fact, many scientists prefer to use the latter.




























0 Response to "41 solute and solvent worksheet"
Post a Comment