41 section 10.3 percent composition and chemical formulas worksheet answers
103 Percent Composition And Chemical Formulas Answer Key 103 Percent Composition And Chemical Formulas Answer Key Percent composition in chemistry typically refers to the percent each element is of the compound's total mass.. The basic equation = mass of element / mass of compound X 100%. For instance, if you had a 80.0 g sample of a compound that was 20.0 g element X and 60.0 g element y then the ... 10.3: Percent Compositions and Chemical Formulas - Quizlet Steps to find empirical formula 1) convert all masses to moles 2) divide each mole value by the smallest number of moles 3) determine the mole ratio and the empirical formula Molecular Formulas give the actual number of atoms of each element in a molecular compound always a whole number multiple of the empirical formula
Chapter10 section03 Percent Composition and Chemical Formulas By Hamd… Chapter10 section03 Percent Composition and Chemical Formulas By Hamdy Karim. May. 21, 2014 • 3 likes • 1,708 views Download Now Download to read offline Education Technology Students will learn about the Percent Composition and Chemical Formulas, also they will learn the difference between the empirical and molecular formulae! Hamdy Karim Follow
Section 10.3 percent composition and chemical formulas worksheet answers
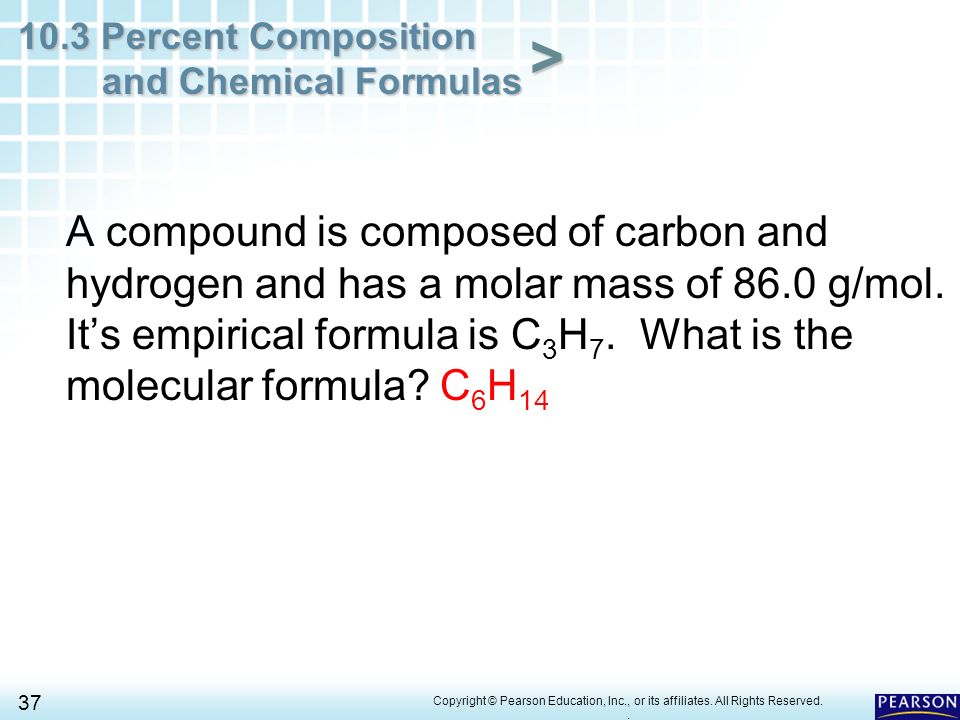
PDF 103 Percent Composition And Chemical Formulas Worksheet Answers As this 103 percent composition and chemical formulas worksheet answers, it ends up subconscious one of the favored ebook 103 percent composition and chemical formulas worksheet answers collections that we have. This is why you remain in the best website to see the amazing book to have. 10.3 Percent Composition and Chemical Formulas - Pittsfield 10.3 Percent Composition and Chemical Formulas 6 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. Percent Composition of a Compound K = 40.3% Cr = 26.8% + O = 32.9% 100% These percents must total 100%. 10.3 Percent Composition and Chemical Formulas 7 Copyright © Pearson Education, Inc., or its affiliates. Chapter 10.3 Percent Composition and Chemical Formulas The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100%. empirical formula a formula with the lowest whole-number ratio of elements in a compound; the empirical formula of hydrogen peroxide (H2O2) is HO The empirical formula of a compound shows
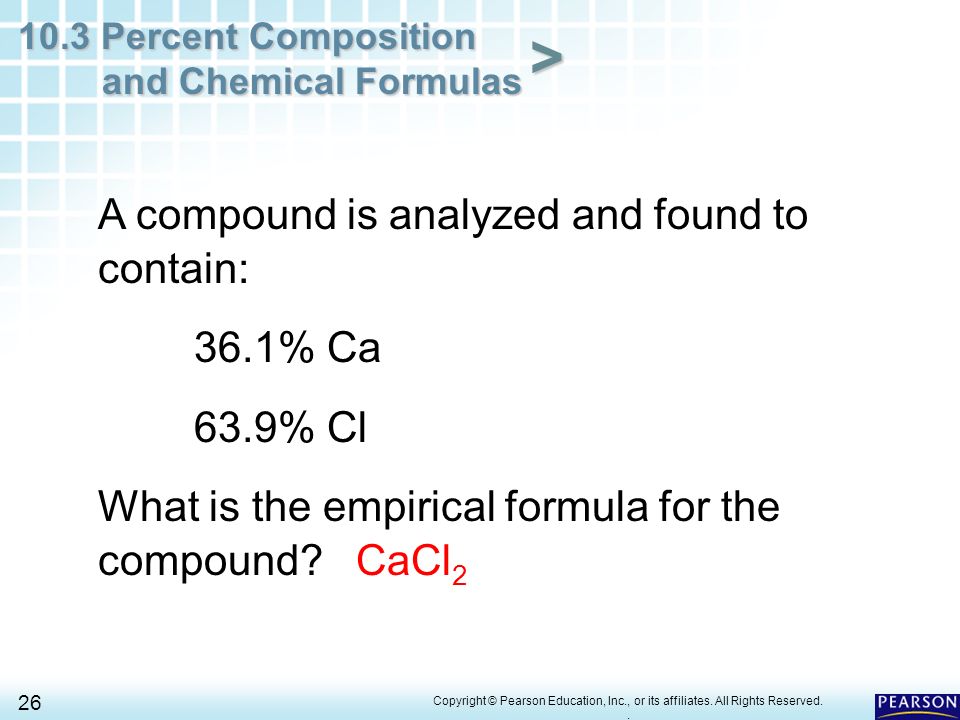
Section 10.3 percent composition and chemical formulas worksheet answers. 10.3 percent composition & chemical formulas answer key/answers section review 10.3 part a completion l percent composition 2. 100 3. molar mass 4. empirical 5. whole-number 6. molecular hi. molar mass fe z 0 3 = vf, 55.8 g fe v 16.0 go 2 rnoife x, ./ + 3 ~o", ~ ~o 1 it~e 1 1.y>11 = 112 g fe + 48 g ° = 160 g fe203 a po 112 g %fe = 0- v x 100 = --- x 100 g fe 2 0 3 160 g part d questions and problems , 104 g … Chapter 10.3 Percent Composition and Chemical Formulas Chapter 10.3 Percent Composition and Chemical Formulas What is the percent composition of a compound formed when 6.85g of magnesium combines with 20.0g of chlorine to form magnesium chloride? What is the percent composition of a compound formed when 2.72g of potassium combines with 2.48g of chlorine to form 5.20g of potassium chloride? 10.3 Percent Composition & Chemical Formulas Answer Key/Answers fSection Review 10.3 Part A Completion L percent composition Hi. molar mass FeZ03 2 rnoiFe x, vf, 55.8 g Fe 1 IT~e . / + 3 ~O", 16.0 gO ~ ~O 1 1.y>11 , 2. 100 3. molar mass 4. empirical 5. whole-number 6. molecular 639~ %Fe 112 g Fe X + 48 g 160 g Fe203 X a po 0 v g Fe203 100 = --- 112 g 160 g = 100 70.0% Fe 447 kg Fe 70.0 leg Fe x 100.kg-FezCJ3 Percent Composition and Chemical Formula | PDF | Mole (Unit) - Scribd f 10.3 Percent Composition and Chemical Formulas It helps to know the percents of the components in a shirt because they affect how warm it is, whether it will need to be ironed, and how it should be cleaned. You will learn how the percents of the elements in a compound are important in chemistry. Slide 6 of 40 End Show
Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 48 Answer 313.6 Work Step by Step H= 124 g/mol Ca (CHO) = 158.1658 H= 124 x 4 = 496 496/158.1658 x100 =313.6 Update this answer! You can help us out by revising, improving and updating this answer. Update this answer PDF 10.3 Percent Composition and Chemical Formulas - Disney II Magnet What is the percent composition of each of the following? a. Cr 2O 3 c. HgS b. Mn 2P 2O 7 d. Ca(NO 3) 2 15. Determine the empirical formula of the compound with the percent composition of 29.1% Na, 40.5% S, and 30.4% O. 16. How many kilograms of iron can be recovered from 639 kilograms of the ore Fe 2O 3? 2 Chapter 10 Chemical Quantities ... Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - Sample Problem 10.12 - Page 331: 39 Answer OH Work Step by Step Each percentage represents the number of grams of an element per 100.0 grams of that compound. Convert these masses to moles using molar masses. 10.3-Percent Composition and Chemical Formulas - Quizlet 10.3-Percent Composition and Chemical Formulas STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by eaecco Terms in this set (8) The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100% TO KNOW Percent composition
10.3 Percent Composition AND Chemical formulas by Nari Moh'd - Prezi Determining the empirical formula of a compound. Convert the percent by mass of each element to moles. Divide each molar quantity by the smaller number of moles to get 1 mol for the element with smaller number of moles. A compound is analyzed and found to contain 25.9% nitrogen and 74.1% oxygen. PDF 103 Percent Composition And Chemical Formulas Worksheet Answers obsession currently. This 103 percent composition and chemical formulas worksheet answers, as one of the most in force sellers here will extremely be in the midst of the best options to review. Now you can make this easier and filter out the irrelevant results. Restrict your search results using the search tools to find only free Google eBooks. PDF 10.3 Percent Composition and Chemical Formulas 10 - Henry County Schools the percent composition or the percent by mass of each element in the compound. The percent composition of a compound consists of a percent value for each different element in the compound. As you can see in Figure 10.13, the percent composition of K 2CrO 4 is K 40.3%, Cr 26.8%, and O 32.9%. These percents must total 100% (40.3% 26.8% 32.9% 100%). PDF 103 Percent Composition And Chemical Formulas Answer Key Read Online 103 Percent Composition And Chemical Formulas Answer Keycome up with the money for each success. next-door to, the publication as well as acuteness of this 103 percent composition and chemical formulas answer key can be taken as capably as picked to act. Now you can make this easier and filter out the irrelevant results. Restrict ...
10.3 percent composition and chemical formulas - Quizlet 10.3 percent composition and chemical formulas Term 1 / 5 How do you calculate the percent composition of compound? C1 Click the card to flip 👆 Definition 1 / 5 The percent by mass of an element in a compound is the number of grams of the elements divided by the mass in grams of the compound, multiplied by 100%. C1 Click the card to flip 👆
Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 49 Answer Emp: C5H10O2 Mol: C5H10O2 Work Step by Step Each percentage represents the number of grams of an element per 100.0 grams of that compound. Convert these masses to moles using molar masses.
5.3 Percent Composition - CHEM 1114 - Introduction to Chemistry Solution. To calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to a percentage: % C = 7.34 g C 12.04 g compound ×100% =61.0% % C = 7.34 g C 12.04 g compound × 100 % = 61.0 %.
PDF 05 CTR ch10 7/9/04 3:29 PM Page 245 PERCENT COMPOSITION AND 10.3 ... It is necessary to know the formula of a compound in order to calculate its percent composition. _____ 8. If the percent by mass of carbon in methane, CH 4, is 75%, then 100 grams of methane contain 25.0 grams of hydrogen. _____ 9. The formula for methane, CH 4, is both a molecular and an empirical formula. _____ 10.
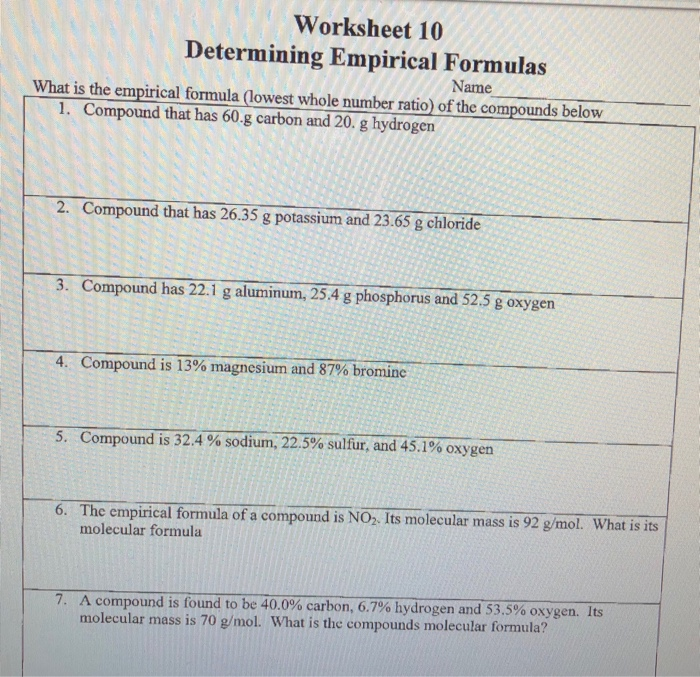
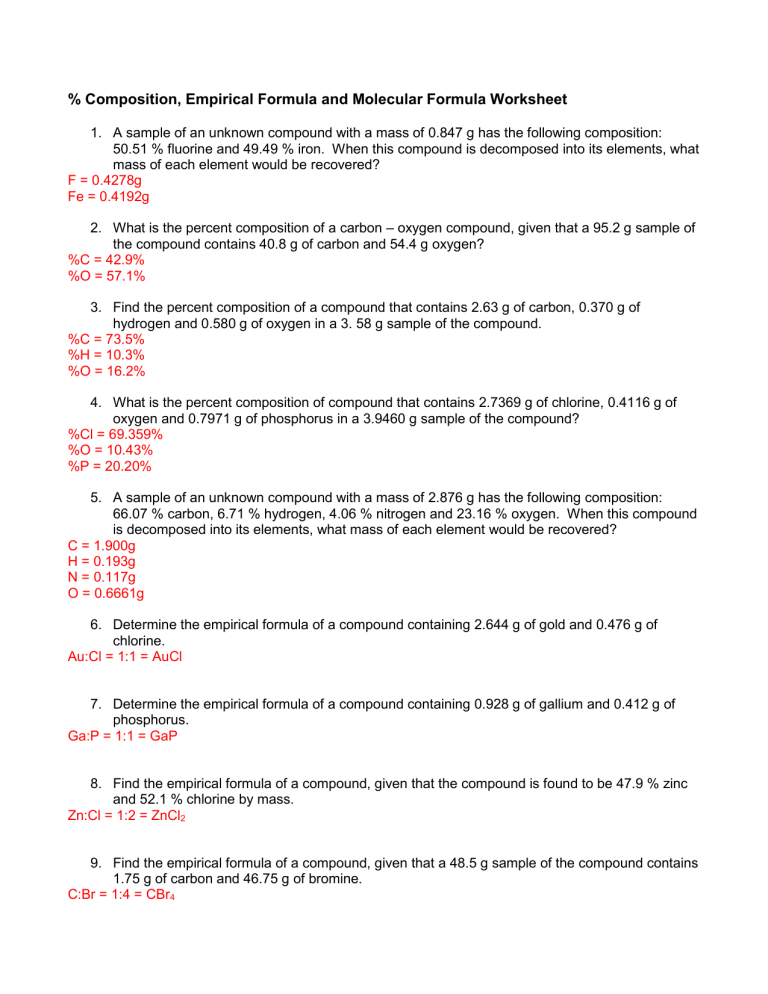
PDF Chapter 10.3 percent composition practice worksheet answers - Weebly What is the percent composition of this compound? _ 2. Find the percent composition of a compound containing 18.35 g of the compound contains 5.74 g of tin, tin and chlorineif:g. '"The __..L element compound.. of acompoundis the percent by mass of each by mass of an element of the element per __ 2 __ the percent in a g by1. 2.
PDF 10.3 percent composition and chemical formulas worksheet answers a. Get! b. CHCI319.8 gPage 2 Slide 1 1 PERCENT COMPOSITION, EMPIRICAL & MOLECULAR FORMULAS Slide 2 2 oNew food labels are required to describe the ingredients using percents of the daily reccom- mended allowance These numbers tell what part of the total # of calories can be obtained from a product AKA percent composition oNew food labels are
Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 44 Answer The empirical formula of a compound shows the smallest whole number ratio of the atoms in the compound. Work Step by Step The empirical formula of a compound shows the smallest whole number ratio of the atoms in the compound.
Chapter 10.3 Percent Composition and Chemical Formulas The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100%. empirical formula a formula with the lowest whole-number ratio of elements in a compound; the empirical formula of hydrogen peroxide (H2O2) is HO The empirical formula of a compound shows
10.3 Percent Composition and Chemical Formulas - Pittsfield 10.3 Percent Composition and Chemical Formulas 6 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. Percent Composition of a Compound K = 40.3% Cr = 26.8% + O = 32.9% 100% These percents must total 100%. 10.3 Percent Composition and Chemical Formulas 7 Copyright © Pearson Education, Inc., or its affiliates.
PDF 103 Percent Composition And Chemical Formulas Worksheet Answers As this 103 percent composition and chemical formulas worksheet answers, it ends up subconscious one of the favored ebook 103 percent composition and chemical formulas worksheet answers collections that we have. This is why you remain in the best website to see the amazing book to have.























0 Response to "41 section 10.3 percent composition and chemical formulas worksheet answers"
Post a Comment